The SH3 domains, found in many different proteins, in diverse numbers and combinations, have relation to deregulated signaling pathways during cancer development and are also associated to other pathologies such as AIDS, osteoporosis or inflammatory processes. Resolving these structures at atomic resolution allows a detailed analysis of their function and features.
Beamline 13-XALOC, devoted to macromolecular crystallography, helped professors Bacarizo and Cámara-Artigas to measure crystals of theses structures at atomic resolution (0.98 Å). X-ray diffraction data was collected using the PILATUS detector.
These structures have been deposited in the Protein Data Bank (PDB) and the research has been published in Acta Crystallographica Section D (Atomic resolution structures of the c-Src SH3 domain in complex with two high-affinity peptides from classes I and II. Julio Bacarizo and Ana Camara-Artigas, Acta Cryst. (2013). D69, 756–766; doi:10.1107/S0907444913001522).
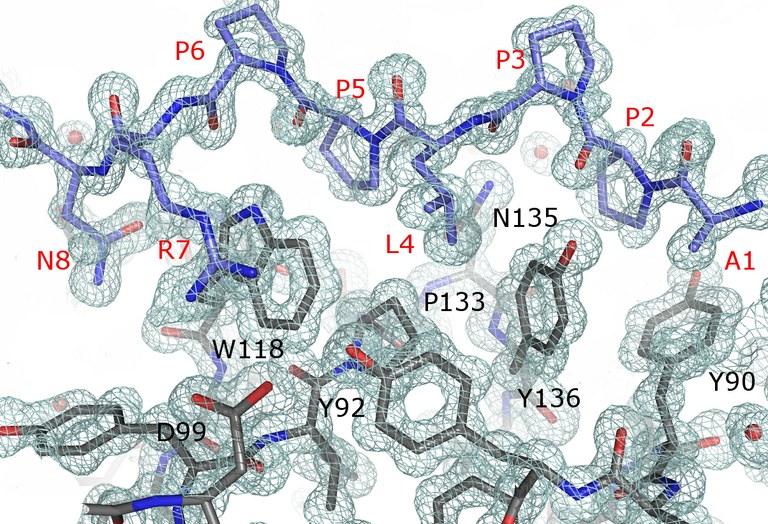
Figure 1. The image shows 2Fo-Fc map contoured at 1.5 σ showing the electron density for the APP12 peptide (in blue, label in red) and residues of the binding site of the Thr98Asp mutant of the c-Src-SH3 domain (in grey, label in black).




