Collage of different protein structures solved at XALOC beamline.
The ALBA Synchrotron has reached one important milestone. After a decade of giving service to the user community, the macromolecular crystallography (MX) beamline XALOC has determined 1,000 structures, and made them public in the Protein Data Bank (PDB). The elucidated macromolecules and macromolecular complexes are involved in a myriad of relevant biological processes and research topics such as drug discovery, diseases or living organisms' functions.
The structures have been unveiled thanks to the powerful X-rays generated at the ALBA Synchrotron light source and the optimized experimental setup of the XALOC beamline, which offers a variety of crystallographic methods, from classical oscillation cryo-crystallography and anomalous diffraction methods to modern time-resolved serial crystallography methods at room temperature.
In particular, the protein structure number 1,000 deposited in the PDB was solved by researchers from the Blas Cabrera Institute of Physical Chemistry from CSIC and other research institutions in the United Kingdom. IP3K, as it is named, is an enzyme that bonds to IP3 protein, a fundamental second messenger in cells, crucial in processes like memory, the immune system or tumoral progression. The results of this research, recently published in Nature Communications, can be very helpful in the design of selective ligands against IP3-binding proteins, opening windows to new therapies in different biological fields, including cancer research.
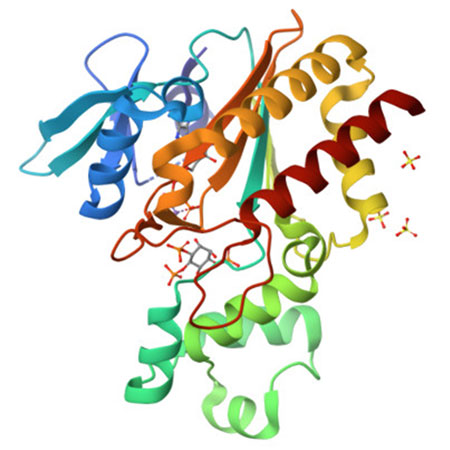
Figure. Image of the ·D structure of IP3K, Inositol-trisphosphate 3-kinase A, protein number 1,000 solved at the XALOC beamline, ALBA Synchrotron.
"Obtaining 1,000 structures in 11 years with one dedicated beamline is a remarkable achievement as it proves the great performance of the instrument and the involvement of our users along these years", says Roeland Boer, scientist in charge of XALOC beamline. "We are very proud of this result that pushes us to maintain and strengthen our commitment to keep serving the community now with a second beamline dedicated to macromolecular crystallography, XAIRA, that will receive the first users by fall 2024", says Judith Juanhuix, head of the Life Sciences section at ALBA.
Shedding light to countless research challenges
Proteins are large and complex molecules that play a key role in living organisms, being involved in most of the cells’ functions. Determining its structure is vital to know more about the insights of life sciences to develop new drugs and therapies.
Some of the proteins solved at the ALBA Synchrotron have been identified as biomarker for diseases like cancer, diabetes and obesity. In other cases they were useful to see how drugs bind to proteins associated to infections like SARS-CoV2, HIV-1 or the disease of the sleeping sickness, among others. And they also helped to explain the inefficacy of some discontinued drugs.
Antibiotic resistance has been better understood studying the high division rate of bacteria or how bacteria share genetic material at the XALOC beamline. Its field of application exceeds the biomedical sector, as it also showed how plants grow and can be designed to resist against disease or severe climate conditions like flood or drought.
XALOC has hosted several industrial experiments aimed at solving the structure of proteins, like the collaboration with Helix Biostructures or Esteve for studying inhibitors against pain.
Mapping the tiniest
X-ray crystallography on biomacromolecules has been extensively used at synchrotron facilities in the last 30 years, and is still evolving to deliver solutions in drug development and dynamics of enzymatic processes of living organisms. The technique takes advantage of the diffraction patterns produced by crystals of macromolecules to determine their structure at near atomic resolution and infer the mechanisms of life.
The high brilliance and tuneability of X-rays generated at synchrotron light sources have made it an extremely efficient tool for structural biology. 90% of all the proteins deposited in the Protein Data Bank have been obtained at synchrotron facilities.
The evolution of this technique in the last years have made possible to collect data remotely and automatically, doing time-resolved experiments and solving structures using ever smaller crystals.


Images of the XALOC beamline at the ALBA Synchrotron. At the left, researcher Fernando Gil from ALBA.




