ALBA Synchrotron
The research was led by Salvador Ventura, from the IBB-Department of Biochemistry at the UAB, and SOM Biotech, a biopharmaceutical company that discovered the use of tolcapone for treating ATTR and holds the patent on it. The crystal structure of TTRtolcalpone was solved by the group of David Reverter, at the IBB-UAB, in collaboration with the XALOC beamline scientists.
ATTR is a rare degenerative disease that mainly affects the nervous system and heart muscle tissue (myocardium), and which is usually passed on from parents to children. It originates when the liver and other areas of the organism produce mutations of the protein transthyretin (TTR), which lose their functional structure. This causes toxic aggregates of amyloid fibres to build up, which, depending on the mutation involved, are deposited in different organs, such as the brain, the kidneys, the nerves, the eyes or the myocardium, causing them to malfunction and bringing on the various forms of the disease. To prevent the disease from progressing, a liver transplant or liver and heart transplant is needed.
Drug repositioning involves taking molecules that have already been approved for a specific therapeutic indication – as is the case with tolcapone for treating Parkinson’s disease – and using them for a different disease, thus quickening their development and patients’ access to new treatments.This strategy also helps lower the cost of treatments, which, in the case of tolcapone, could facilitate its administration in countries like Brazil and Portugal, where the polyneuropathic variant is highly prevalent. The drug has been designated an orphan drug for ATTR by the American Food and Drug Administration. This is important, as in the United States there is a large group of people suffering from the cardiomyopathic variant of ATTR.
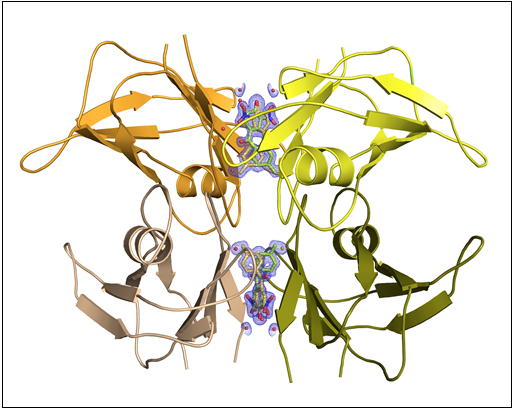
In the study, the researchers conducted biophysical trials – in vitro in cell cultures and ex vivo in human plasma and in mouse models of the disease – to show that tolcapone is a powerful inhibitor of the aggregation of amyloid fibers by TTR, stabilizing the structure of the protein and thus slowing down the advance of the disease. This is a hitherto unknown property of the drug, which is used to treat Parkinson’s disease. The compound turns out to be four times more effective than the only medicine currently available for treating the polyneuropathic variant of ATTR. The results were positive for all variants of the disease that were studied: familial amyloid polyneuropathy and cardiomyopathy (which affects the peripheral nerves and the myocardium, respectively) and senile systemic amyloidosis, a sporadic form that appears in a very high percentage of men over 60 years of age (and also affects the myocardium). In addition, the treatment was shown to cross the blood-brain barrier, making it the first to tackle the variants that affect the central nervous system. Tolcapone acts by imitating the process by which the thyroid hormone – T4 or thyroxine – binds to TTR in the bloodstream. Just like the hormone, the drug binds closely to the protein, tying together the four protein sub-units that form the protein’s structure. This binding has been proven to stabilize the protein, preventing the sub-units from separating and then forming aggregates. The structural details of the interaction of tolcalpone with the transthyretin (TTR) tetramer, performed in collaboration with XALOC beamline scientists of the ALBA Synchrotron, revealed the molecular details of this strong drug-target interaction.

Fig.: Drawing depicting the transthyretin tetramer (TTR) in complex with Tolcalpone (in stick representation with the electron density maps). Based on deposited structure with PDB code 4d7b.
Tolcalpone has been described as a powerful drug to advance in the fight against familial amyloidosis. This molecule has proved in preclinical trials to be up to four times more effective than the only pharmacological treatment currently available for familial transthyretin amyloidosis, a rare degenerative disease, and has already been tested in a clinical trial with patients. It acts as a powerful inhibitor of the protein deposits that cause the disease, thus slowing its advance.
Reference
: