ALBA Synchrotron
A team from the Materials Science Institute of Madrid -CSIC leads an international research that synthetized a zeolite with extra-large pores by expanding and connecting silica chains. This material has applications in water and gas decontamination and catalysis. Experiments carried out at the MSPD beamline of the ALBA Synchrotron had a key role in determining the structure of the zeolite.
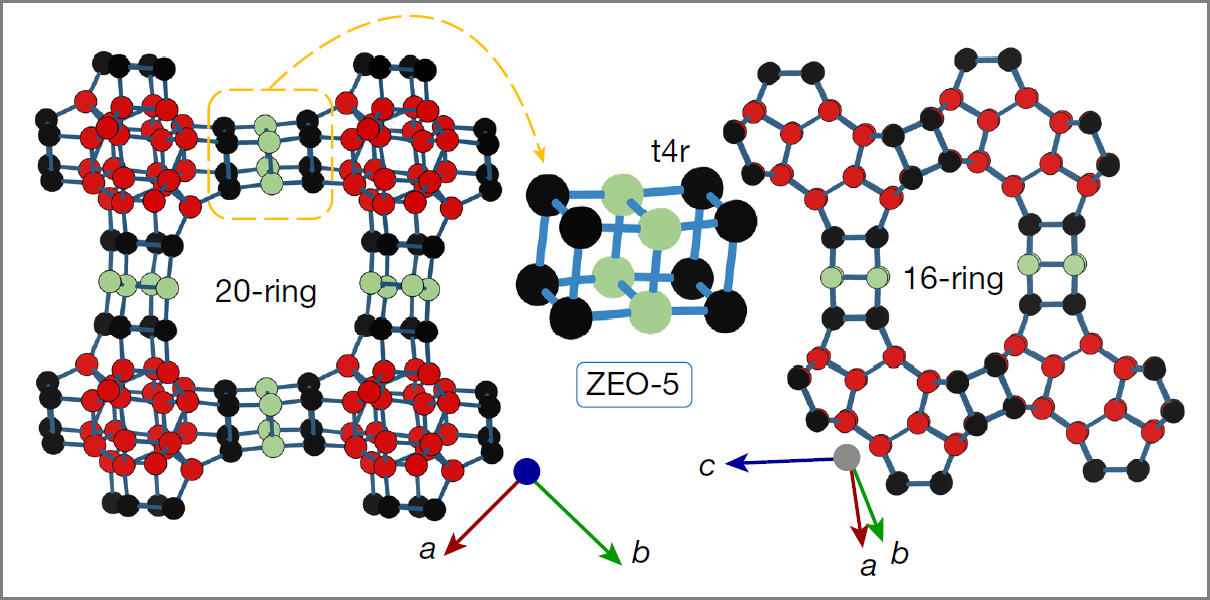
A team from the Materials Science Institute of Madrid (ICMM-CSIC) leads an international research that has succeeded in creating the world's most porous zeolite. The study, published yesterday in the journal Nature, opens up new avenues for water and gas decontamination and "demonstrates that it is possible to make more porous materials that are stable," says Miguel Camblor, researcher at the ICMM-CSIC and lead author of the study.
Zeolites are microporous crystalline silicates. These are materials with applications in decontamination, catalysis, gas adsorption, and cation exchange. For decades, obtaining stable zeolites with greater porosity and, therefore, capacity for absorption and processing of large molecules, has been an important scientific goal. However, this is not a simple challenge: "until recently, it challenged our synthetic capacity," indicates Camblor.
The team already developed in recent years two zeolites with "extra-large" pores in the three spatial directions that also exhibited high stability. On this occasion, they have created a stable aluminosilicate zeolite with extra-large pores open through rings of more than 12 tetrahedra, capable of processing even larger molecules.
"The structure of this zeolite presents unprecedented characteristics and demonstrates that with different methods, things that were believed impossible can be found, such as this world record in porosity," highlights Camblor, who indicates that they have already used the zeolite for the absorption of volatile organic compounds.
To determine the structure of the zeolite, the research team has combined electron diffraction techniques and powder X-ray diffraction, the latter available at the MSPD beamline of the ALBA Synchrotron. The X-rays produced at the ALBA’s accelerator provided crucial information on the position of the atoms in the zeolite structure.
Furthermore, by adding titanium to the material, it has been observed that it can function as a catalyst in the oxidation of propylene with cumene hydroperoxide, a process that leaves no residues and is 100% selective. It is better than conventional catalysts, which makes this zeolite very promising in relevant industrial sectors.