ALBA Synchrotron
Researchers from the ALBA Synchrotron and the Universitat Autònoma de Barcelona (UAB) have analysed with synchrotron light different Alzheimer's aggregates, their location and their effect in cultivated neuronal cells. Results, published in Analytical Chemistry, pave the way to better understand the development of this disease that affects more than 30 million people worldwide.
Cerdanyola del Vallès (Barcelona) – 15th February 2018
. Memory loss, communications' difficulties, personality and behaviour changes, orientation problems ... Unfortunately, these symptoms are widespread in our society, since 30 million people worldwide and 1.5 in Spain suffer from the effects of Alzheimer's, according to the World Health Organization (WHO) and the Spanish Confederation of Family Members of Alzheimer's and other dementias patients (CEAFA), respectively. Alzheimer's is the most important cause of dementia and is described as a multifactorial disease that leads to neuronal cell death. Nowadays, there is no effective treatment to fight against or to prevent it.
When a person has Alzheimer's, amyloid plaques are generated inside his brain. They are made of deposits or aggregates of the amyloid beta peptide. This peptide - which comes from a protein that is necessary for cellular functioning - tends to be aggregated by adopting different sizes and morphologies, depending on the physical and chemical conditions around it. Although it is already known that the presence of the beta amyloid peptide, together with other factors such as oxidative stress, play a key role in the onset and development of the Alzheimer's disease, it is not still clear what causes the disease and what the consequences are.
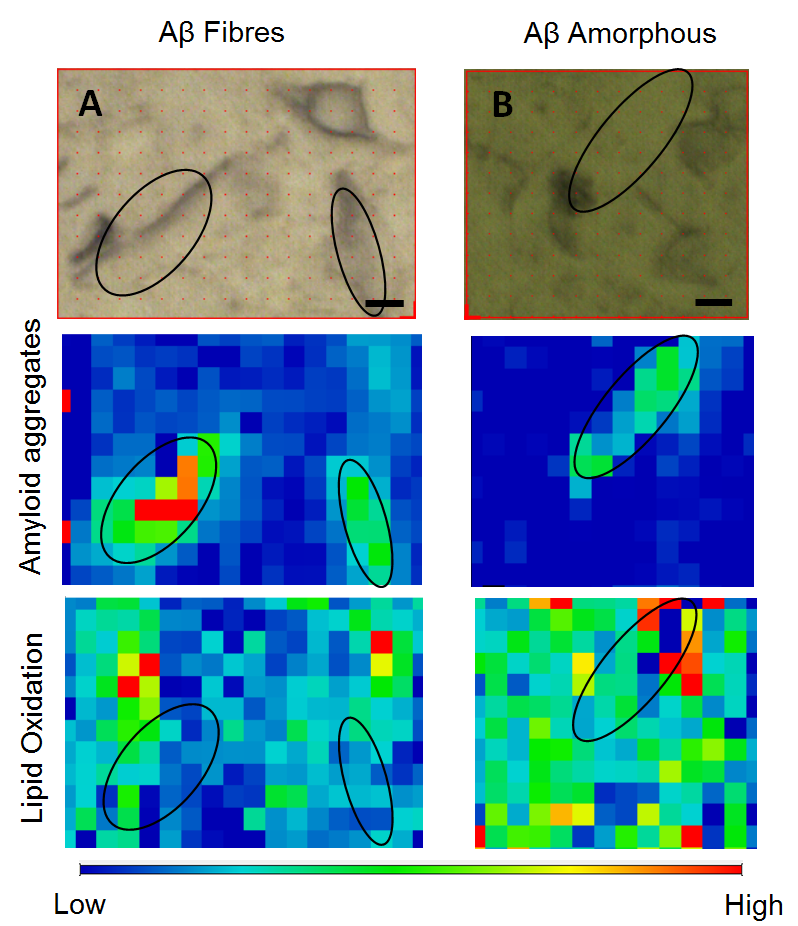
In order to know more about the mechanisms behind the toxicity of proteins and amyloid peptides, researchers from the ALBA Synchrotron and from the Biophysical Unit of the Universitat Autònoma de Barcelona (UAB, Alzheimer's Structural Biology group), in collaboration with scientists from the European Synchrotron Radiation facility, have carried out experiments with infrared light in Barcelona and Grenoble. Infrared light is absorbed by the molecules bonds, enabling to know the chemical composition of a sample. Connecting a microscope to the infrared light, scientists can also find its chemical distribution. Thanks to the great brightness of the synchrotron light, this distribution can be measured at very high spatial resolutions. Scientists have analysed the effect of two types of amyloid peptide aggregates (fibrillar and amorphous) on cultured neuronal cells. The results indicate that the amorphous aggregates cause a higher degree of oxidation and they have obtained, for the first time, the in situ co-localization of these aggregates in cultured cells. The results are consistent with previous research conducted with brain samples, where it was found that oxidation was higher in the regions that surrounded amyloid plaques and supported the hypothesis that amyloid peptides are the cause of cell oxidation, the hallmark of Alzheimer's disease.
This study, led by Josep Cladera from the UAB, is a step forward in the characterization of these amorphous aggregates and in their consideration as potential toxic agents in the onset and development of the disease. "This work is consistent with the hypothesis that amyloid peptides, especially in non-fibrillar forms, are capable of oxidizing cells, a key point for the development of the disease. Thanks to the infrared light, not only we can analyse the effects of these aggregates, but also we can relate them with the structure of peptides when they generate different aggregates. This can be of great importance for identifying the toxic causes of the disease", says Núria Benseny, researcher from the at the ALBA Synchrotron.

Fig.: Visible images and infrared map of the analyzed aggregates of amyloid peptide (fibrillar and amorphous) in cultured neuronal cells. Black lines are drawn in order to mark the position of the peptide aggregates. Color scale (10 µm) corresponds to intensity: blue, low intensity; red, high intensity.