ALBA Synchrotron
In October 2013, a friendly users group, formed by Celia Rogero, Jorge Lobo-Checa and Mikel Abadía from the Centro de Física de Materiales (CSIC-UPV/EHU) and Donostia International Physics Center, performed at the Near Ambient Pressure Photoemission branch an experiment of attraction/liberation of dioxygen by phthalocyanine molecules.
The main aim of the experiment was to understand the reactivity of biological molecules in contact with metal surfaces. The experiment was fully performed inside the NAPP UHV chambers (sample cleanness, molecular deposition and surface analysis at different pressures).
The adsorption and self-assembly of biological species on solid surfaces can provide important information to understand basic biological reactions, such as one of the most important reaction in living organisms: the breathing. This process consists on the transport and storage of the dioxygen/carbon dioxide molecules thanks to haemoglobin formed by Fe tetrapyrrole molecules. In order to mimic this process at CIRCE beamline we expose Fe-phthalocyanine molecules, which were evaporated in vacuum on a clean Au(111) surface, to different
O2
partial pressures. By means of a combination of XPS and NEXAFS we detected the anchorage of
O2
when pressure in the chamber was changed from UHV to around
1x10-2
mbar, being this process reversible. Thus, when the
O2
partial pressure was reduced again
O2
liberation takes place.
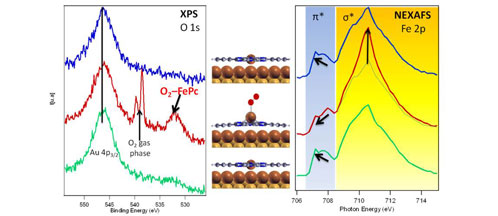
This reversibility was basically detected by analyzing the O 1s core level photoelectron spectra and the Fe 2p adsorption edge of the system under different pressure conditions inside the chamber. When pressure is around 10-2 mbar, a new component appears in the O 1s core level region in the XPS spectrum. This new component is completely separated from the
O2
in the gas phase, disappears when the gas is removed and was not observed for the clean Au(111) under the same pressure conditions. Because of these reasons, it is possible to associate it with the
O2
molecules bonded to the Fe ions in the FePc molecules. Moreover, Fe 2p adsorption edge reveals that Fe(II) ions change their conformation during
O2
bonding. The central metal core of the phthalocyanine moves from the initial planar configuration to a more tetrahedral structure when the
O2
is temporary bonded to the surface. This change is again reversible, i.e. the system returns to the flat configuration when the gas is removed. Similar conformational changes have been reported in living organisms for the haemoglobin during the breathing process. Thus with this system we confirm that it is possible to mimic these biological processes at the nanoscale.

O1s XPS spectra (left) and Fe 2p adsorption edges (right) of FePc/Au(111) measured at a base pressure of 7x10-10mbars (blue spectrum), during O2 partial pressure of 10-2mbars (red spectrum) and after removing the O2, measured at pressure of 1x10-9mbars (green spectrum). Schemes illustrate the conformational of the molecules due to the bond between Fe ions and O2 molecules.