ALBA Synchrotron
Sodium-sulfur batteries are promising electrical energy storage technologies that can serve as a key solution to intermittency problems and can be integrated with renewable forms of energy generation. An international research team has reported the synthesis of micro-mesoporous carbon nanospheres with continuous pore distribution as an efficient sulfur host for sodium-sulfur batteries.
The work sheds new light on the progress of the sulfur cathode in sodium-sulfur batteries and provides a promising strategy for the viable design of other metal–sulfur batteries. Experiments at the CLAESS beamline in ALBA allowed determining the sulfur species during charge/discharge processes.
Solar and wind power are useful resources for energy generation but they are intermittent (at night or on cloudy days solar panels do not work, for example). Electrical energy storage technologies serve as a key solution to these intermittency problems and can be integrated with renewable forms of energy generation. Among these technologies, room-temperature sodium-sulfur (Na–S) batteries are deemed to be one of the most promising candidates, owing to their high theoretical energy density – the amount of energy they can store - and low cost. Nonetheless, this battery system suffers from a slow reaction rate at room temperature, which radically limits battery performance and makes difficult its practical commercialization.
An efficient strategy to deal with this challenge is the use of porous carbon material as a host to encapsulate molecular sulfur, significantly enhancing its conductivity. This system acts as the cathode of the battery, which is the electrode where reduction occurs. To make the battery work, sodium ions have to migrate from the anode to the cathode. However, in these systems, it is a challenge to provide fully accessible sodium ions that do not obstruct the sub-nanosized pores of the carbon host.
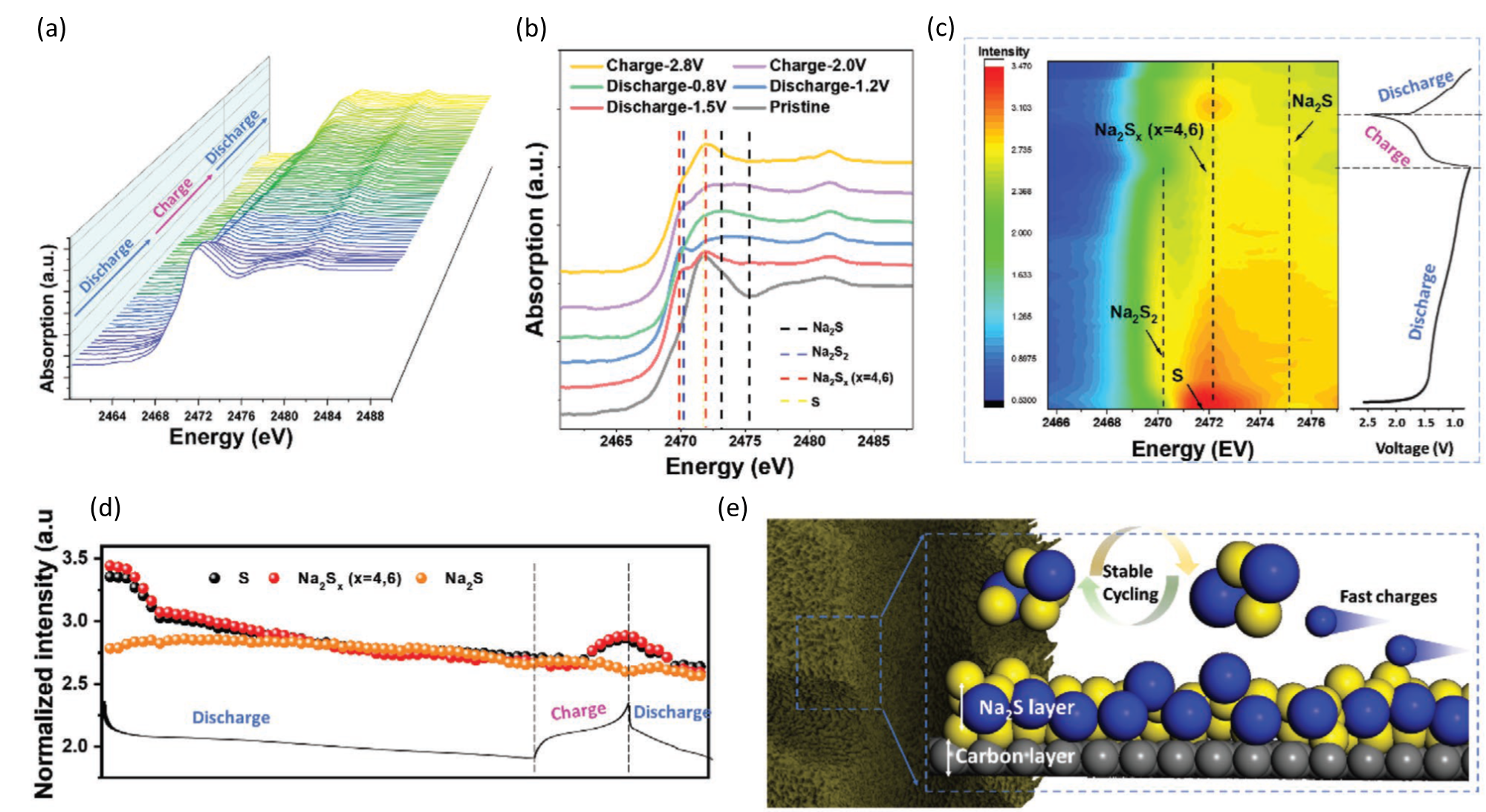
In a publication in the Advanced Materials journal, an international research team made up of Australian and Chinese institutions in collaboration with the ALBA Synchrotron has reported the synthesis of micro-mesoporous carbon nanospheres (MMPCS) with continuous pore distribution as an efficient sulfur host for sodium-sulfur batteries. This unique feature creates continuous channels that allow the movement of sodium ions without channels being obstructed. This enables a high conductivity, leading to fast sulfur reduction-oxidation reaction during the charge/discharge processes.
The work sheds new light on the progress of the sulfur cathode in room-temperature sodium-sulfur batteries and provides a promising strategy for the viable design of other metal–sulfur batteries, especially lithium-sulfur and potassium-sulfur batteries.
The team synthesized three different samples of the porous carbon spheres and carried out various experiments in order to analyze and compare the characteristics and behavior of each sample. More specifically, they determined the sulfur speciation by Operando sulfur K-edge X-ray absorption spectroscopy (XAS) at the CLAESS beamline of the ALBA synchrotron. These experiments, together with in-situ X-ray diffraction (XRD) data collected at the Australian Synchrotron and discrete Fourier transform (DFT) calculation results proved that a stable sodium sulfide interphase exists in the porous carbon spheres samples synthesized at 800°C and plays a key role in improving sodium-ion diffusion rate and cycling stability.
This work is a collaboration between the Kunming University of Science and Technology (China), the University of Wollongong (Australia), the ALBA Synchrotron, the University of New South Wales (Australia), the University of Technology Sydney (Australia), the Australian Synchrotron and the Wenzhou University (China).
With the collaboration of Fundación Española para la Ciencia y la Tecnología. The ALBA Synchrotron is part of the UCCs of the FECYT and has received support through the FCT-20-15798 project.