ALBA Synchrotron
An international team of researchers has restudied the hydration of alite, the main component of ubiquitous Portland cement. Using a multitechnique approach including synchrotron Pair Distribution Function analysis, they have been able to show that C-S-H gel is composed of defective clinotobermorite nanoparticles within a monolayer calcium hydroxide soup. The results are published today in Scientific Reports.
Cerdanyola del Vallès, 4th June 2018.
Tricalcium silicate (alite), the main constituent of Portland cement, hydrates to produce crystalline calcium hydroxide (portlandite) and calcium-silicate-hydrates (C-S-H) nanocrystalline gel. This heterogeneous reaction, originally studied by Le Chatelier, is well known at the microscale but poorly understood at the nanoscale. In this work, a multitechnique approach, including synchrotron X-ray powder diffraction, has been employed to quantitatively follow alite hydration process: i) its dissolution, ii) portlandite crystallization and iii) C-S-H gel precipitation. It has been confirmed that C-S-H gel has (CaO)1.8SiO2(H2O)4.0 average composition microscale. Chiefly, the analysis of synchrotron pair distribution function (PDF) data collected (see Fig. 1) has shown that that C-S-H gel is heterogeneous at the nanoscale being composed of defective clinotobermorite, with Ca11Si9O28(OH)2.8.5H2O average composition, and monolayers of Ca(OH)2.
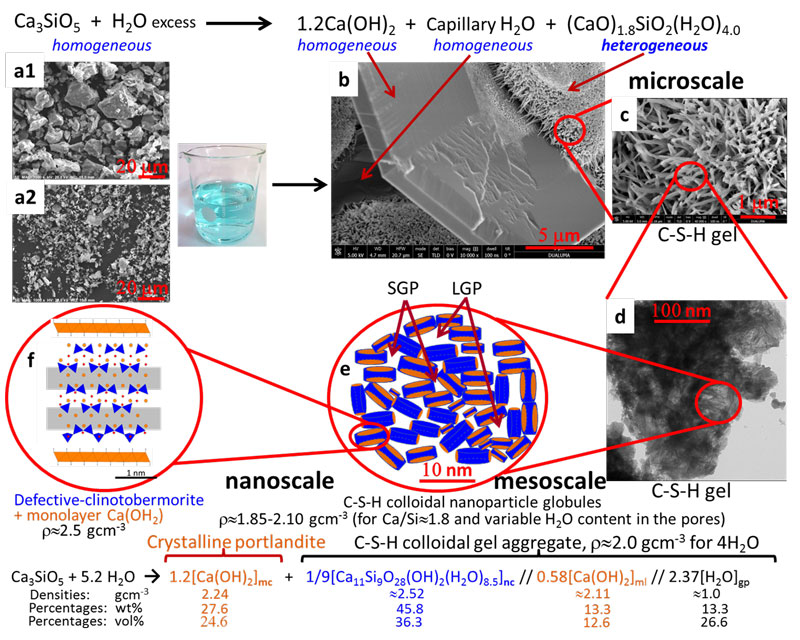
From these results and observations from electron microscopy and previous reports, researches lead by ALBA Synchrotron, with contributions from University of Malaga (Spain), University of Milan (Italy) and Østfold University College (Norway) have proposed a new multiscale model for the hydration of alite (see Fig. 2) which explains the observed mass densities and Ca/Si atomic ratios at the relevant scales. At the nanoscale, below 10 nm, C-S-H gel is composed of a fine intermixing of defective clinotobermorite, particle sizes ranging 3-5 nm with Ca/Si ratio close to 1.2 and r»2.5 gcm-3, and monolayers of Ca(OH)2, r»2.1 gcm-3. The calcium silicate component justifies the previously reported nanoglobules density, r»2.6 gcm-3. These aggregates generate the gel pores. At the mesoscale, between 10 and 100 nm, neat C-S-H gel appears with variable compositions, Ca/Si ratio and water content, centred at (CaO)1.8SiO2(H2O)4.0. This is explained by slightly different defective clinotobermorite to Ca(OH)2-monolayers local ratios and the variable gel pore water. At the microscale, above 100 nm, heterogeneous (CaO)1.8SiO2(H2O)4.0 gel and homogeneous portlandite are arranged enclosing volumes of water, termed capillary water. The multiscale picture reported in this work should be taken into account for developing theoretical models (see Fig. 2).
The new model explains previously ununderstood cement behaviours. For instance, it justifies a striking feature of the hydration of cements blended with fly ash, where the measured portlandite content decreases much less than predicted by current thermodynamic modelling. Although portlandite is still present, the Ca/Si ratio in C-S-H decreases from close to 1.8 in plain pastes to close to 1.4 in fly ash blends. This is now explained by the consumption of Ca(OH)2 monolayer component of the C-S-H gel in the pozzolanic reaction. This new model also explains another noticeable behaviour of Portland cement pastes. Current thermodynamic modelling predicts that all crystalline portlandite should carbonate before the C-S-H, but experiments showed that they carbonate simultaneously. This is now explained as heterogeneous C-S-H gel contains Ca(OH)2 monolayer at the nanoscale that reacts with CO2 to yield poorly crystalline CaCO3.
The challenge of this work has been to prove the existence of amorphous Ca(OH)2 (monolayers) in the presence of portlandite, i.e. crystalline-Ca(OH)2. This could be a more general behaviour as amorphous calcium carbonate coexists with calcite, etc.

Figure 1: PDF refinements. Experimental (blue circles), fitted (red lines) and difference (grey lines) PDF patterns for alite paste (a) from 40 to 70 Å; (b) from 10 to 25 Å; and (c) from 2 to 10 Å. For details of the fits, the readers are referred to the original publication.

Figure 2: Multiscale picture of alite hydration reaction at different length scales. (Top) Alite hydration reaction at the microscale. (a1) SEM micrograph for C3S_21µm. (a2) SEM micrograph for C3S_3µm. (b) FEG-SEM micrograph for C3S_21µm paste showing a homogeneous portlandite plate microparticle, voids arising from capillary water, and three agglomerates of heterogeneous C-S-H gel. (c) Enlarged view of one C-S-H gel region in (b). (d) TEM micrograph for an alite paste showing interspersed foil-like C-S-H nanoparticles at the mesoscale. (e) Schematic representation of the C-S-H colloidal nanoparticles of clinotobermorite (blue) and monolayer Ca(OH)2 (orange) generating the small gel pores and large gel pores. (f) Schematic representation of a single C-S-H nanoglobule composed by defective clinotobermorite and two monolayers of Ca(OH)2 at the nanoscale. (Bottom) Alite hydration reaction of alite at the nanoscale, highlighting the three main components of colloidal C-S-H nanocomposite. The approximate densities, mass and volume percentages of the different components are also given for an overall water content of four water molecules per silicate.
Acknowledgements: This work has been supported by Spanish MINECO through BIA2014-57658-C2-2-R, which is co-funded by FEDER, BIA2014-57658-C2-1-R and I3 (IEDI-2016-0079) grants.