ALBA Synchrotron
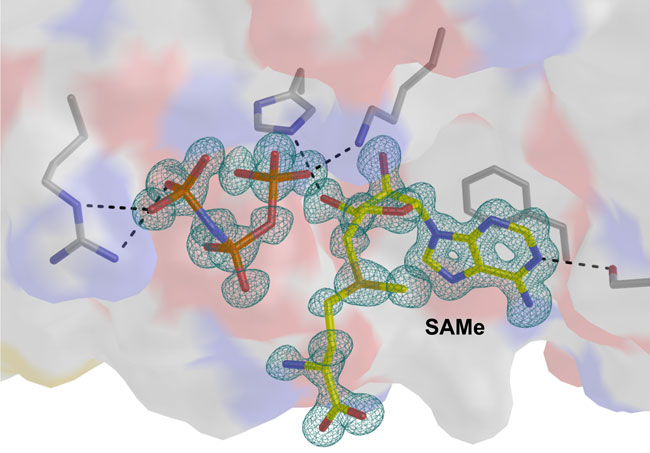
A team of scientists from CIC bioGUNE, University of Liverpool and Cedars-Sinai hospital in California, using high-resolution structures, have determined the catalytic steps of the enzyme MATα2. This study, with data collected at the ALBA Synchrotron, opens up a new pathway in the development of highly targeted drugs that act exclusively on this enzyme, regulating cancer cell growth.
This enzyme sensitizes SAMe, a molecule that plays a key role in the correct functioning of the cells. In normal cells, SAMe is produced by a homologue protein MATα1. However, in colon and liver cancer cells, this molecule is produced by MATα2. This switch of function alters the levels of SAMe in the cell, promoting the reproduction of cancer cells.
CIC bioGUNE researchers have for the first time obtained a group of crystallographic structures in which various stages of the enzymatic reaction MATα2 can be observed, thereby providing a detailed insight into the mechanism of catalytic action. This information will enable the future development of molecules that can be used to regulate the function of this enzyme.
Part of the research was done in European light sources with beamlines dedicated to X-ray crystallography like XALOC, in the ALBA Synchrotron, and I04-1, in Diamond (UK).
The CIC bioGUNE researcher Adriana Rojas, one of the co-authors of this study published in the Proceedings of the National Academy of Sciences USA (PNAS), highlights the relevance of this result. "The fact that we can see the crystallographic structure at 1 angstrom resolution provides us with information of an extremely precise nature for the next step: the development of drugs designed to block or reduce the activity of this protein".
This work is part of the research line of José María Mato, General Director of CIC bioGUNE, and his team, who have dedicated more than 30 years of research to the field of liver cancer.
