ALBA Synchrotron
Using ALBA and ESRF X-rays, researchers were able to solve the structure of the fibre head of turkey adenovirus 3 (TAdV-3), which causes fatal hemorrhagic enteritis in turkeys.
Adenoviruses cause mild diseases (“flu” or “colds”), but also more serious afflictions. At the same time, they are under investigation as vectors for gene and cancer therapy, and used as vaccination agents. Adenoviruses are icosahedral, with a trimeric fibre inserted into each pentameric vertex. The fibre protein interacts with the host cell receptor through its distal head domain. The Adenoviridae family contains five genera: Mastadenovirus, Aviadenovirus, Atadenovirus, Siadenovirus and Ichtadenovirus. Mastadenoviruses infect mammals, including humans, while aviadenoviruses infect birds. Siadenoviruses have been isolated from frog, tortoises and birds. The genus takes its name from a specific open reading frame similar to bacterial sialidases.
Turkey Adenovirus 3 (TAdV-3) is a siadenovirus. Virulent TAdV-3 causes fatal hemorrhagic enteritis in turkeys, but avirulent strains are suitable vaccines. The TAdV-3 fibre protein has 454 residues, of which amino acids 304-454 make up the head domain. This head domain was expressed, purified and crystallised. Crystallographic data collection for the avirulent version of the TAdV-3 fibre head domain and a selenomethionine derivative was performed at the European Synchrotron Radiation Facility beamline ID14-EH4, with the help of Andrew McCarthy. X-ray diffraction data for the virulent version fibre head crystals were collected at XALOC beamline of ALBA with the help of Jordi Benach, Fernando Gil and Jordi Juanhuix and for the ligand-bound forms at BM30 of the ESRF. All crystals were isomorphous and belonged to space group I23. De novo structure solution was by single-wavelength anomalous dispersion (SAD). This is the first siadenovirus for which the structure of the fibre head has been determined.
Curiously, the TAdV-3 fibre head structure is more similar to reovirus fibre heads than to other adenovirus fibre heads. Each monomer contains beta-sandwich, in which the C-strand is interrupted by a beta-hairpin “arm” contacting a neighbouring monomer. This arm is a unique feature. The structures of avirulent and virulent forms are virtually identical. To identify possible receptors, glycan microarray profiling was performed by Michelle Kilcoyne and Lokesh Joshi of the National University of Ireland

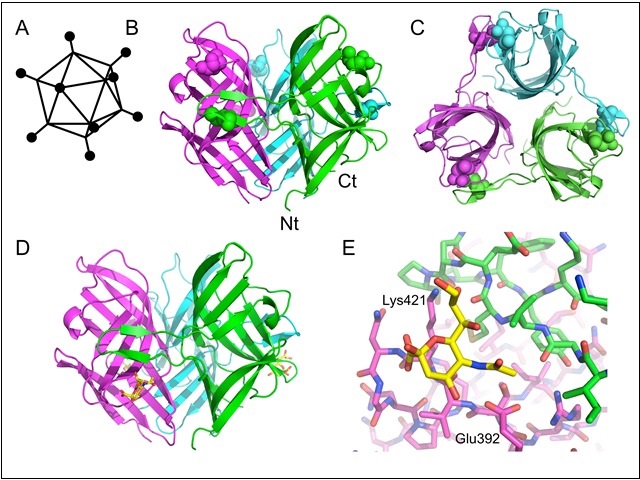
Fig: Structure of the TAdV-3 fibre head. A) Schematic structure of an adenovirus. The black balls represent the receptor-binding fibre head domains. B) Side view of the fibre head trimer. C) Top view of the fibre head trimer. D) Structure of the TAdV-3 fibre head protein bound to 3'-sialyllactose (stick model, carbons yellow). E) Close-up of the binding site for sialyllactose on the fibre head. The two interacting residues that were mutated are labelled. Panels B, C, D and E are adapted from figures published in the journal reference.
We also soaked crystals with sialyllactose. A binding site on the side of the molecule, just below the beta-hairpin, was identified and confirmed by sitedirected mutagenesis. The binding site is constituted by six residues from one monomer, and three from the beta-hairpin of the neighbouring monomer. Because the binding affinity is low, the natural receptor may well be a more complex sialylated cell surface molecule, interacting more extensively with the fibre head. Knowledge of the structure and receptor-binding properties of the TAdV-3 fibre head may facilitate the design of chimeric adenoviruses and improved vaccination or gene therapy vectors.
This work was performed in collaboration with Monika Ballmann, Mária Benkő and Bálasz Harrach from the Institute for Veterinary Medical Research in Budapest, Hungary.
Reference
: "Structure and Sialyllactose Binding of the Carboxy-Terminal Head Domain of the Fibre from a Siadenovirus, Turkey Adenovirus 3." Singh AK, Berbís MÁ, Ballmann MZ, Kilcoyne M, Menéndez M, Nguyen TH, Joshi L, Cañada FJ, Jiménez-Barbero J, Benkő M, Harrach B, van Raaij MJ. (2015) PLoS One 10(9): e0139339. DOI: 10.1371/journal.pone.0139339. PMID: 26418008.