ALBA Synchrotron
Researchers at the CNIO, the CRG and the IBMB-CSIC solve a key problem in biology: how human cells build their microtubules. During cell division, microtubules are responsible of distributing the genetic material to each daughter cell.
Sample preparation and microtubules high-resolution image acquisition were performed at the IBMB-CSIC’s Electron Cryomicroscopy Platform, located at the Joint Electron Microscopy Center (JEMCA), inside the ALBA Synchrotron. The work published in Science lays the groundwork for future breakthroughs in the treatment of diseases ranging from cancer to neurodevelopmental disorders.
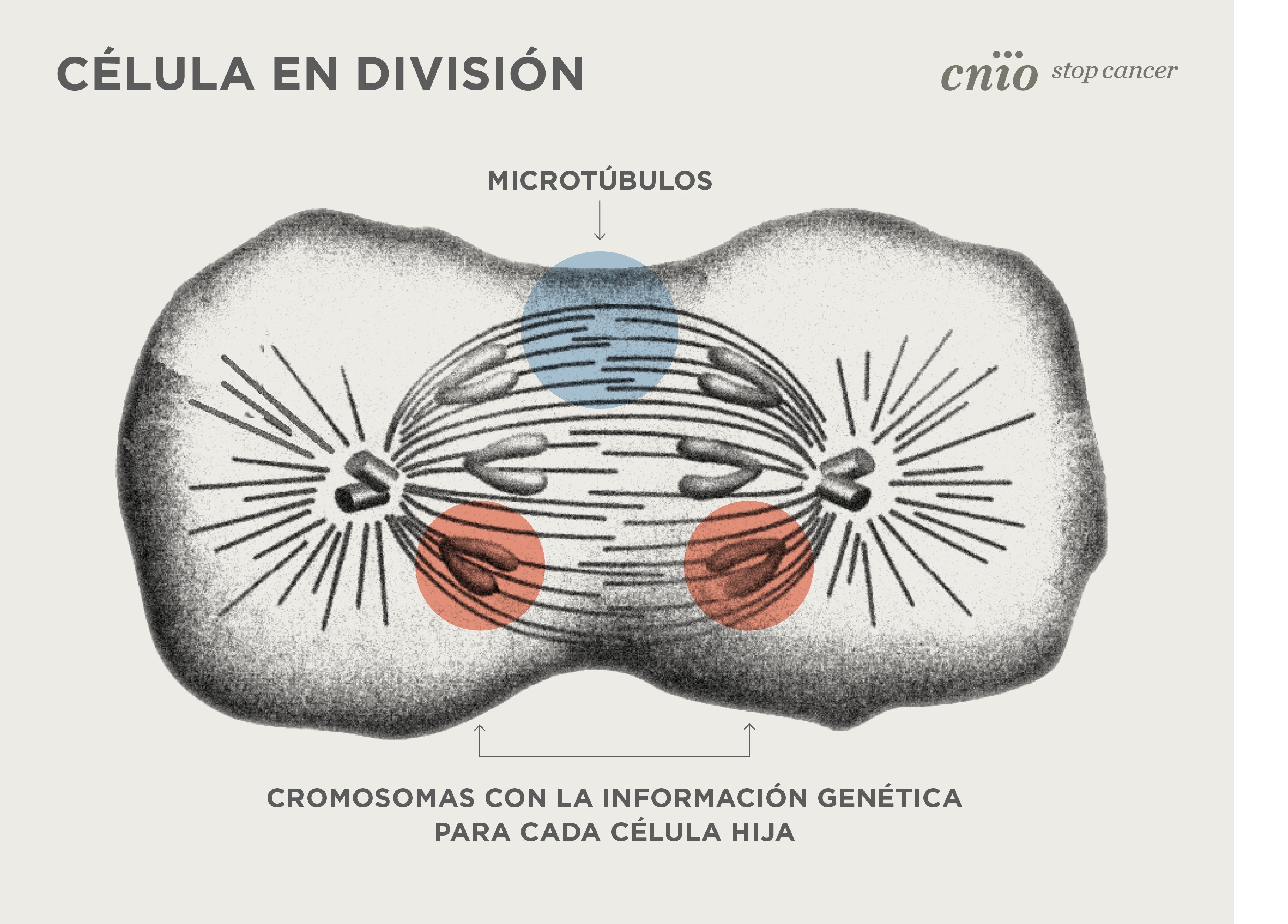
Cells in the human body are constantly dividing. With each division the genetic information contained in the chromosomes is duplicated, and each daughter cell receives a complete copy of the genetic material. It is a sophisticatedprocess, a clockwork mechanism that involves refined and fast changes within the cell. To make this possible, the cell relies on microtubules, tiny structures that are indeed tube-shaped. The high-resolution images, now obtained at the Basque Resource for Electron Microscopy (BREM) and the microscope Cryo-TEM at JEMCA (microscopy platform located at ALBA), answer a question that has been hanging in the air for years: how microtubule formationbegins in the early stages of cell division.
For the first time, researchers at the Centre for Genomic Regulation (CRG), the Spanish National Cancer Research Center (CNIO) and the Institute of Molecular Biology of Barcelona (IBMB-CSIC) have succeeded in making the equivalent of a film showing how human cells initiate the construction of their microtubules. The findings, published yesterday in the journal Science, solve this problem brought up years ago and thus contribute in the treatment of diseasesranging from cancer to neurodevelopmental disorders.
When cell division begins, the chromosomes move to the center of the cell. Then, the cell quickly sprouts from its two ends large tubes that hook the chromosomes and pull each of the copies towards the two poles of the cell. "That’s why we say that microtubules play a key role in cell division. We need to understand very well the mechanisms that trigger the formation of these microtubules, at the right place and at the right time." explains Óscar Llorca, researchers at the CNIO.
Microtubules act also as "cellular highways" for moving cellular components between different areas of the cell. They are also structural elements that shape the cell itself, among other tasks. "Microtubules are critical components of cells. Here we capture the process in action inside human cells. Given the fundamental role of microtubules in cell biology, this could eventually lead to new therapeutic approaches for a wide range of disorders." adds Thomas Surrey, CRG researcher.
Microtubule formation begins when a complex structure made up of several proteins, and called gTuRC ("gammaturc"), closes, forming a ring. The new work unveils the mechanism by which gTuRC closes into a ring and becomes a perfect mold, capable of launching microtubule formation. The closure of gTuRC occurs when the first molecular piece of a microtubule gets attached to it. "As soon as this first brick enters, a region of gTuRC is able to hook it and, like a loop, acts as a latch that pulls the ring closed and launches the process", explains Llorca.
One million frames in a movie at atomic scale
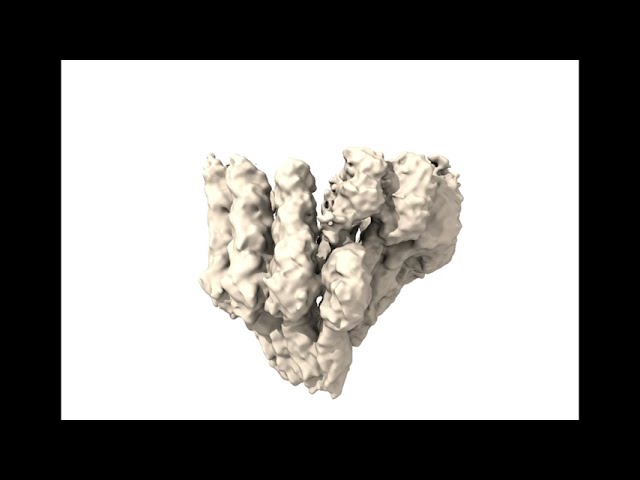
Visualizing this process required purifying gTuRC from human cells and reproducing the microtubule initiation process in the test tube. Thanks to cryo-electron microscopy, the research team imaged over a million microtubules.
First, the microtubules under construction were observed at the IBMB-CSIC’s Electron Cryomicroscopy Platform, located at the , inside the ALBA Synchrotron. "They were frozen in a thin layer of ice, preserving the natural shape of the molecules involved," explains Pablo Guerra, head of this Platform. That’s how the best experimental conditions for observing microtubules in formation were determined. The best frozen samples were then sent to BREM for imaging, and the resulting images were transferred to the CNIO for analysis and determination of the three-dimensional structures at atomic resolution.
To analyse such amount of images and data, artificial intelligence techniques were used. "In practice, having more than a million microtubules in different stages of growth is equivalent to having many frames of a movie in high resolution. You "just" have to arrange them in the right order to see the movie in progress." explains Marina Serna, scientist from CNIO.
The result are three-dimensional structures at atomic resolution that represent the different stages of how the construction of a microtubule begins, and how the gTuRC ring becomes the mold that launches the formation of microtubules.

First steps of microtubule building on the gTuRC ring, visualized through cryo-electronic microscopy (cryo-EM). View from the side and from above (bottom left). Credit: Marina Serna/CNIO
Implications for health
This study offers a basic knowledge useful for learning how to correct errors in the functioning of microtubules, which are associated with cancer, neurodevelopmental disorders and other conditions ranging from respiratory problems to heart disease. The next step is understanding regulation: deciphering how microtubule formation is controlled by yet-to-be-found regulators in cells. "Several candidates have been described in other studies, but their mechanism of action is unclear. Further work, clarifying how regulators bind to gTuRC, may transform our understanding of how microtubules work, and eventually offer alternative sites that one might want to target to prevent cancer cells from going through the cell cycle," Surrey concludes.
The first steps of microtubule nucleation. Credit: Marina Serna/CNIO
Text adapted from the CNIO piece of news.
Work in the Surrey lab was supported by the Spanish Ministry of Science and Innovation to the EMBL partnership, the Centro de Excelencia Severo Ochoa and the CERCA Programme of the Generalitat de Catalunya, and by the Francis Crick Institute, which receives its core funding from Cancer Research UK, the UK Medical Research Council, and the Wellcome Trust. T.S. acknowledges also support from the European Research Council and from the Spanish Ministry of Science and Innovation. C.B. was supported by EMBO and a Marie Curie fellowship. Work in the Llorca lab was funded by the Agencia Estatal de Investigación, Ministerio de Ciencia e Innovación, and co-funded by the European Regional Development Fund (ERDF-UE); O.L. laboratory also had the support from the National Institute of Health Carlos III to CNIO. The IBMB-CSIC CryoEM Platform is supported by project Project, IU16-014045 from the Generalitat de Catalunya and by “ERDF A way of making Europe”, by the European Union.