ALBA Synchrotron
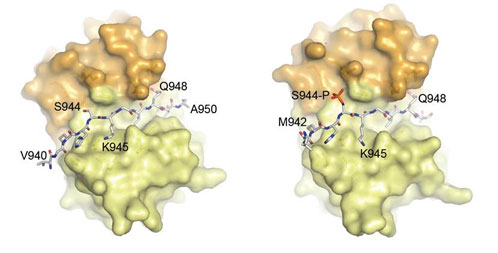
The structures obtained at XALOC correspond to the complexes of LC8 homodimers (yellow and orange) with the phosphorylated (right, PDB ID 3ZKF) and non-phosphorylated (left PDB ID 3ZKE) versions of a Nek9 peptide.
Nek9/Nercc1, Nek6, and Nek7 constitute a signaling module activated in early mitosis and involved in the control of spindle organization. Previous experiments suggested that LC8 binding to Nek9 was regulated by Nek9 autophosphorylation on Ser944, a residue immediately located N-terminal to the LC8 conserved (K/R)xTQT binding motif, and that this was crucial for the control of signal transduction through the Nek/Nek6/7 module.
The crystal structures obtained at BL13-XALOC of LC8 with both Nek9 peptides, together with different biophysical experiments, explain the observed diminished binding affinity of Nek9 to LC8 upon phosphorylation on Ser944 within the Nek9 sequence, thus shedding light into a novel phosphorylation regulatory mechanism that interferes with LC8 protein-protein complex formation.
The accepted manuscript can be found here:
http://www.jbc.org/content/early/2013/03/12/jbc.M113.459149.full.pdf+html
