ALBA Synchrotron
An international group of researchers has unveiled the transformation processes that take place during synthesis of lithium-rich layered oxides that are used as cathode materials in lithium-ion batteries. These low-cost materials showed very high capacity and speed, becoming promising candidates for the development of the electric vehicles and energy storage systems. Using synchrotron light, researchers were able to determine structural and chemical changes during the synthesis of these materials.
Lithium-ion batteries are very popular in many of the electronic devices we used every day. They are charged and discharged when lithium ions travel back and forth between the anode and cathode inside the battery. The bigger amount of lithium ions the cathode has, the greater capacity of storing energy the battery has.
Most of the current lithium-ion batteries' cathodes are made of lithium and cobalt, an expensive and poisonous element. Moreover, these systems are limited in their performance, using only about 50% of the total material capacity. So, this research topic has attracted the interest of the scientific community in order to find new ways to develop cobalt-free lithium-rich cathodes.
Now, a group of researchers from the Karlsruhe Institute of Technology, the Research Centre Jülich, the Technical University of Munich (Germany), the Sichuan University (China), the University of Wollongong (Australia) together with scientists from DESY and ALBA Synchrotron facilities have developed a technology for synthesis of lithium-rich layered oxides.
"These compounds are a promising candidate to be used as cathode in the next generation of lithium-ion batteries because they operate at high voltages and deliver high capacities. That means that smaller batteries could deliver more energy. However, their cycle life remains limited, reducing their applications. Their electrochemical performances are strongly related to the phase composition and preparation procedure. This is why this type of studies are very relevant", says Björn Schwarz, researcher at the Karlsruhe Institute of Technology.
Scientists have proposed a scalable method for the synthesis of these alternative cathode materials: a coprecipitation route followed by a microwave heating process. Apart from having a detailed characterisation of the process, the experiment showed that these cobalt-free cathode materials have very high capacity at a high speed, about 10 times faster than conventional batteries.
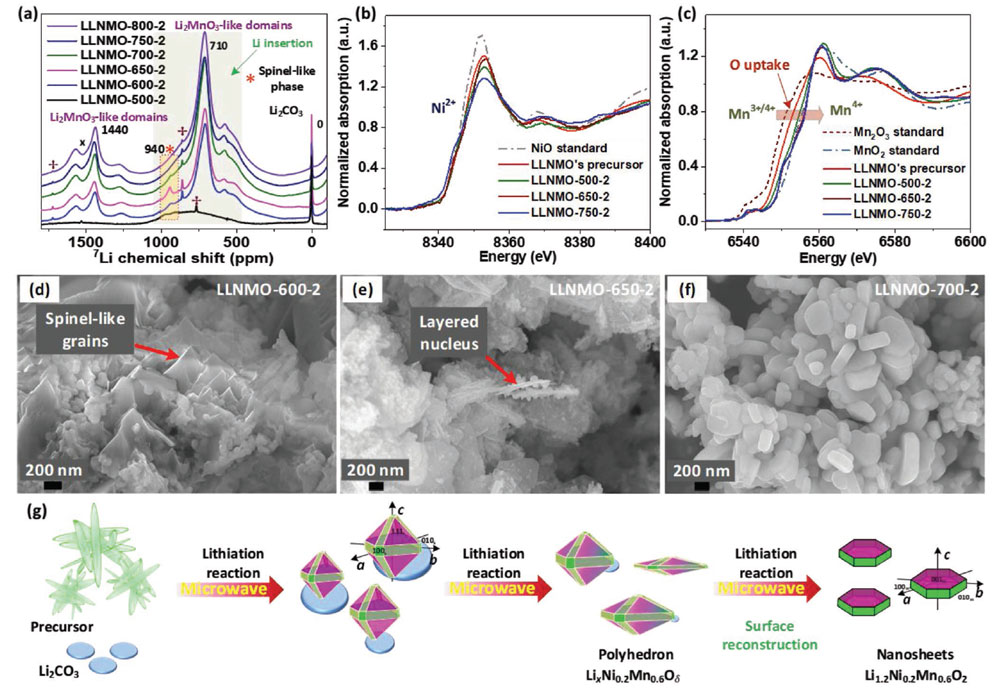
Several synchrotron light techniques were used at the ALBA Synchrotron and DESY to comprehensively understand the transformation of precursors during the formation of the lithium-rich cathode materials.
"Synchrotron light has been very useful to follow the reaction in real time and determine how their structure changes", says Aleksandr Missiul, post-doctoral research fellow at the and co-author of the publication. "At the same time, X-ray absorption spectroscopy at the was able to identify the chemical changes occurring during the synthesis", says Laura Simonelli, co-author and beamline responsible of CLAESS.
MSPD has become a leading beamline in Europe for this kind of studies and complementarity between MSPD and CLAESS is clearly an asset. X-ray measurements were also done at PETRA III in DESY. Scanning electron microscopy, nuclear magnetic resonance spectroscopy and electrochemical studies completed the study.
The results have been published in Advanced Energy Materials Science News, featured in the front cover of the magazine.
"Understanding how these cathodes react will enable to engineer future cathode materials for the next-generation lithium ion batteries", says Weibo Hua, researcher at the Karlsruhe Institute of Technology.
This research has been supported by CALIPSOplus that has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 730872