ALBA Synchrotron
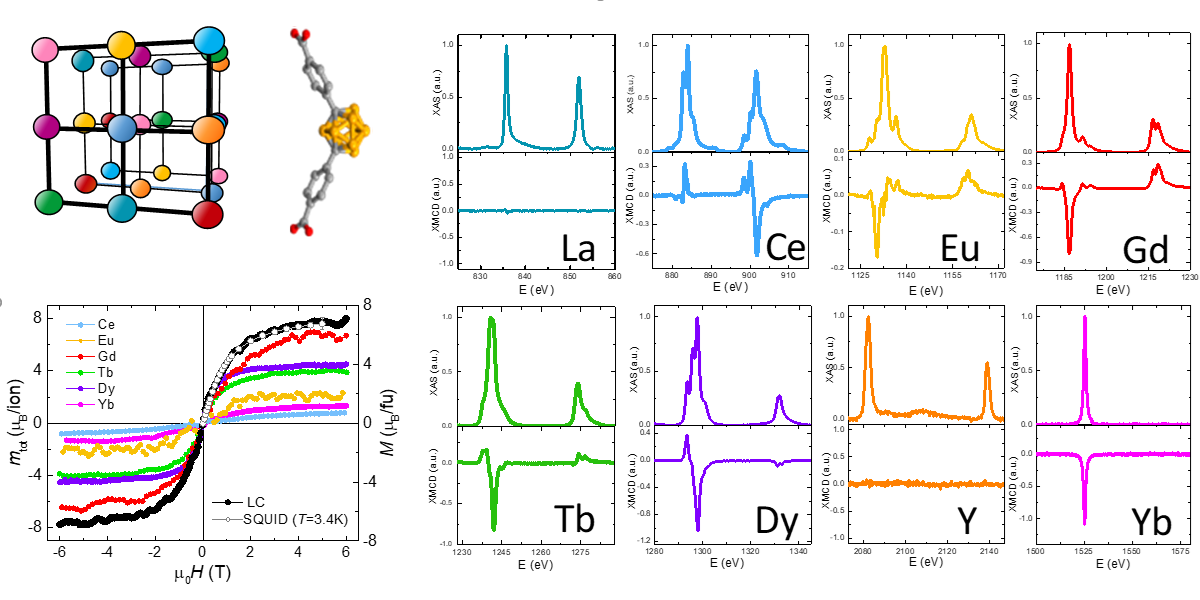
A team of international researchers from Spain (ICMAB, EUSS, ICN2, INMA, ICMM, ALBA) and UK (University of Southampton) have discovered a method to synthesize carborane-based Metal Organic Frameworks (MOFs) incorporating any desired combination of multiple rare earth ions. The optical, thermal and magnetic properties of a proof-of-concept multi-metallic MOF including 8 different rare earth ions have been reported. Element-selective XAS & XMCD spectroscopy at BOREAS beamline of ALBA was used to characterize the individual magnetic properties of the 8 ions.
This innovative approach allows for multifunctionality à la carte, and this newly discovered multi-metallic MOFs could have potential applications in fields such as quantum computing, luminescence, anticounterfeiting and thermometry.
Multi-metallic multivariate rare earth (RE) metal−organic frameworks (MOFs) are of interest for the development of multifunctional materials, with potential application in fields such as quantum computing, luminescence, anticounterfeiting or thermometry. However, up to now, examples with more than three RE cations were rare and obstructed by compositional segregation during synthesis. Now, a team from ICMAB-CSIC, EUSS and coworkers has developed a method for the synthesis of carborane-based MOFs incorporating any desired combination of different RE ions in nearly equimolar amounts. The remarkable potential of this strategy was demonstrated through the synthesis and characterization of the magnetic, thermal and optical properties of the first ever MOF containing eight RE ions: Dysprosium (Dy), Terbium (Tb), Gadolinium (Gd), Cerium (Ce), Ytterbium (Yb), Europium (Eu), Lanthanum (La), Yttrium (Y). Element-selective X-ray absorption spectroscopy (XAS) & X-ray Magnetic Circular Dichroism (XMCD) spectroscopy at BOREAS beamline of ALBA was used to characterize magnetically each of the individual RE ions incorporated in the MOF structure.
Across various domains, the investigation of materials incorporating multiple metal elements is essential, as the combination of different metal cations often leads to the emergence of novel or improved properties. In the field of alloys, the advent of a new “compositionally complex material” designs including 5 or more transition metals has opened a flurry of research interest from all over the world. However, the addressable combination of multiple metal elements has remained a major synthetic challenge in the field of MOFs.
MOFs are a type of materials constructed through the combination of organic linkers and metal ions into periodic, extended structures. In the particular case of multi-metal multivariate (MTV) MOFs, different metal elements occupy topologically equivalent positions in the secondary building units (SBUs), without altering the network connectivity as compared to that of their single-metal counterparts. All these materials manifest an increased complexity, which can provide a new generation of materials with diverse properties However, the greater the number of elements to be combined, the more challenging and difficult is their judicious incorporation in the MOF structure. Such complexity has been overcome in the case of transition-metals, where MOFs including up to ten different elements have been reported. However, the synthesis of multi-metalic RE-based MOFs has been elusive and, up to now, only instances combining two or three cations of similar sizes existed.
Now, a team from ICMAB-CSIC and EUSS has demonstrated the synthesis of a MM-MTV RE MOF incorporating eight different RE ions with different sizes and in nearly equimolar amounts (mCB-8RE). The MOF is formed by a combination of RE cations (La, Ce, Eu, Gd, Tb, Dy, Y, Yb) and a 1,7-di(4-carboxyphenyl)-1,7-dicarba-closo-dodecaborane (mCB-L) linker (Figure 2). The steric bulkiness and acidity of mCB-L is crucial for the incorporation of different size RE ions into the MOF structure. Demonstration of the incorporation of all RE cations was performed via compositional, structural, optical, thermal and magnetic characterization.
The susceptibility-temperature product, and field-dependence of the magnetization of the mCB-8RE agree with the predictions for a diluted system of non-interacting ions, considering the MOF elemental composition. Ac susceptibility reveals the material exhibits field-induced magnetic relaxation through a thermally-activated process and a direct relaxation mechanism. Moreover, the compound shows interesting, magnetocaloric effect performance, as shown by heat capacity measurements performed down to 0.3 K.

Figure 2. Crystal structure and homogeneous metal mixing in mCB-8RE. (a) Crystal structure of single-metal mCB-Ce, showing the construction of the 3D structure from the rod-shaped SBUs and the carborane linker mCB-L. Blue and yellow polyhedra represents the Ce coordination spheres and icosahedral closo-carboranes, respectively. H atoms are omitted for clarity. Color code: B, orange; C, gray; O, red; N, dark blue; Ce, blue. Three independent metal atoms (SBU) are repeated along the structure to provide 1D inorganic rod-shaped chains that are bridged by the carborane linker to form the observed 3D structures; (b) PXRD patterns of experimental mCB-Ce, mCB-8RE and simulated mCB-Ce, demonstrating phase purity and isostructurality; (c) SEM and EDX analysis of RE cations at two indicated positions (A and B) on a crystal, and on a different crystal, d.
XAS and XMCD performed at BOREAS was used to characterize spectroscopically each of the RE ions (Figure 1), and determine their orbital, spin and total magnetic moments through application of the XMCD sum. The magnetic moment per formula unit of the mCB-8RE compound, determined from the linear combination of the magnetic moments obtained by XMCD(H) for each RE, is in very good agreement with the magnetization curve M(H) measured by SQUID at the same temperature, within experimental This study beautifully illustrates the power of element-selective XMCD technique to investigate the magnetic response of individual ions within a material.
This work paves the way for the synthesis of multi-metallic MTV-MOFs with different combinations of RE cations selected à la carte, for fundamental research and the development of multifunctional materials with customized magnetic, optical and thermal properties. These molecular systems are of interest for application in various fields, such as luminescence, anticounterfeiting, magneto-refrigeration or thermometry. Moreover, the preparation of multi-metallic RE MOF materials, enabled now by the new synthetic approach, could help to advance in the scaling of molecular systems for Quantum Computing.
This work was a multidisciplinary collaboration between the Institut de Ciència de Materials de Barcelona (ICMAB-CSIC), EUSS School of Engineering, Instituto de Nanociencia y Materiales de Aragón (INMA-CSIC), Catalan Institute of Nanoscience and Nanotechnology (ICN2), the Materials Science Institute of Madrid-Spanish National Research Council (ICMM-CSIC), University of Southampton and staff at BOREAS beamline in ALBA Synchrotron.