ALBA Synchrotron
An international research team has demonstrated the oxidation effect of HOPG at room temperature by the deposition of an ultra-thin layer of cobalt monoxide (CoO). The formation of carbon defects reveals a weakening of the C-C sigma bonds, facilitating the carbon gasification reaction catalyzed by CoO at lower temperatures than using typical metallic nanoparticles. This fact opens the door to a more comprehensible and efficient graphite and graphene nanopatterning for multiple applications. The results were obtained using in-situ NAP-XPS measurements at the NAPP endstation of the CIRCE beamline in the ALBA Synchrotron.
Nanopatterning of graphitic substrates, as highly oriented pyrolytic graphite (HOPG) and graphene, has become a hot research topic due to the multiple applications these materials could have in numerous technological fields. Nevertheless, the atomic scale mechanisms behind these nanofabrication processes are still mostly unknown, which hinders the control and potential applications of these novel nanostructures.
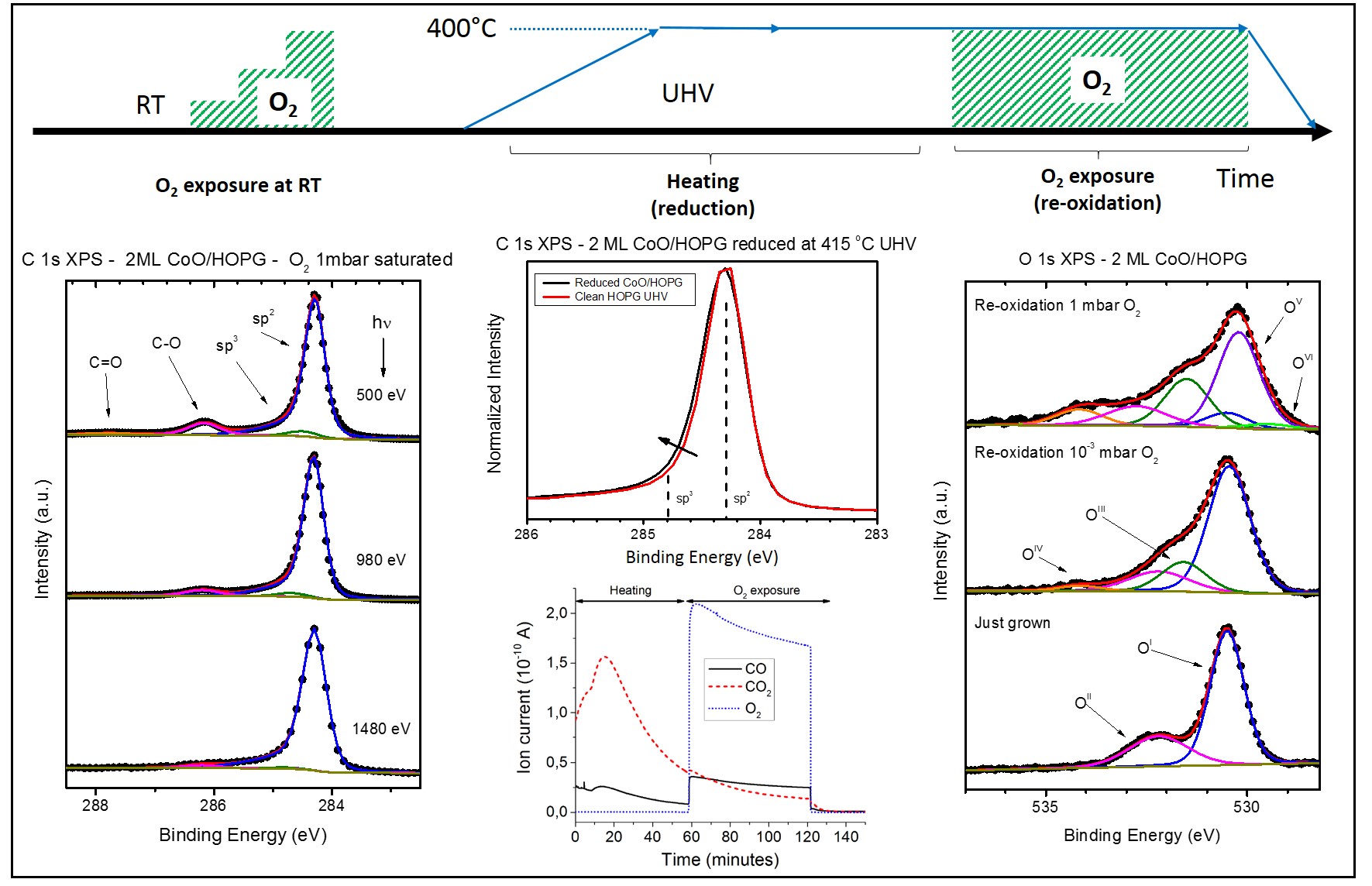
Using in-situ Near-Ambien Pressure X-ray Photoelectron Spectroscopy (NAP-XPS), a team of researchers led by the Laboratory of Coatings and Nanostructures of the Applied Physics Department and Nicolás Cabrera Materials Science Institute (Universidad Autónoma de Madrid, Spain) in collaboration with the ALBA Synchrotron, the Instituto de Cerámica y Vidrio-CSIC (Spain), the Instituto de Ciencia de Materiales de Madrid-CSIC (Spain) and the Molecular Foundry of the Lawrence Berkeley National Laboratory (USA) has demonstrated the oxidation effect of HOPG at room temperature by the deposition of an ultra-thin layer (2 equivalent monolayers) of cobalt monoxide (CoO). The subsequent reduction of this layer - at 400 °C and under ultra-high vacuum conditions (UHV) - to metallic Co nanoparticles and posterior oxygen (O2) exposure leads to the formation of carbon defects that reveal a weakening of the C-C sigma bonds, facilitating the carbon gasification reaction catalyzed by CoO at lower temperatures - at least ~200 °C less - than using typical metallic nanoparticles. This fact opens the door to a more comprehensible and efficient graphite and graphene nanopatterning at lower temperatures for multiple applications.
A qualitative model of the reaction mechanism was stated from the in-situ measurements performed at the NAPP endstation of the CIRCE beamline.
Monitoring the changes with synchrotron light
The complete process consists of three stages:
1) Deposition of 2 equivalent monolayers of CoO and O2 exposure at room temperature,
2) CoO reduction into Co nanoparticles at 400 °C in UHV and
3) CoO re-oxidation at 400 °C under O2 atmosphere.
Changes on the HOPG surface were tracked in-situ, demonstrating a close relationship between the initial morphology of the CoO deposit – which consists of the formation of a wetting layer followed by the growth of islands - and the oxidation of the graphite uppermost layers. Precisely, this oxidation of HOPG at room temperature is responsible for the weakening of the C-C sigma bonds (detected by XPS under different O2 pressures), and ultimately responsible for the low-temperature nanopatterning.
Changes on the Co were also followed. From the metallic reduced state (2nd stage), when the system was exposed to oxygen at 400 °C (start of the 3rd stage), the metallic Co nanoparticles were immediately re-oxidized to CoO, which converts to a CoO/Co3O4 mixture as the O2 pressure is increased. XPS Co 2p3/2 spectra showed a variation of the relative intensity of the two characteristic satellites of the spinel, corresponding to Co2+ and Co3+. The strong variations in oxidizing/reducing ambient conditions together with the high mobility of cations throughout the surface supports this initial Co2+/Co3+ionic inter-exchange until the equilibrium is reached.
The intermediate states of the carbon gasification reaction were also studied by monitoring the O1s core level, under different O2 pressures and photon energies (different depth probes). A final qualitative reaction model is proposed taking into account these results in combination with mass spectroscopy measurements, where traces of carbon monoxide (CO) and carbon dioxide (CO2) were detected during O2 exposure as byproducts of the reaction.
Moreover, ex-situ Atomic Force Microscopy (AFM) and Kelvin Probe Force Microscopy (KPFM) measurements showed the appearance of two more novel nanostructures in addition to nanochannels: nano-rings and nano-strips. The creation of defects on the HOPG surface may modify its reactivity and hydrophobicity, facilitating the self-assembling of molecules forming nanostrips along the armchair axis of graphite. The graphite defects density is greater around the re-oxidized cobalt oxide nanoparticles, allowing the folding of these strips into nano-rings.
In-situ XPS measurements under operando conditions and with tuned photon energy can only be performed in NAP-XPS systems installed in synchrotron facilities, such as the one in ALBA. Besides, the identification of different components separated by small amounts of energy can only be carried out if very-high-energy resolution is guaranteed.
With the collaboration of Fundación Española para la Ciencia y la Tecnología. The ALBA Synchrotron is part of the of the of UCC network from the FECYT and has received support through the FCT-20-15798 project.