ALBA Synchrotron
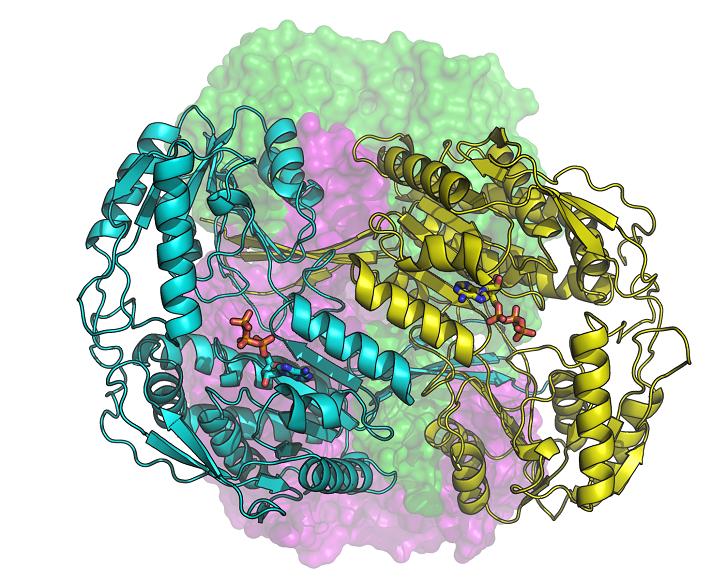
Human aldehyde dehydrogenase 1A3 (ALDH1A3) is an enzyme that binds ATP, as well as a biomarker of tumor-initiating cells responsible for resistance to chemotherapy. According to a study by the UAB Department of Biochemistry and Molecular Biology in collaboration with the ALBA Synchrotron, their silencing restores the sensitivity of tumor cells to chemotherapeutic agents.
Enzymes are catalytic proteins that accelerate specific biological reactions. Human aldehyde dehydrogenase 1A3 (ALDH1A3) belongs to a group of enzymes that specialize in eliminating aldehydes, which are highly reactive and often toxic compounds, and are involved, among other things, in the metabolic pathway of alcohol as well as in hormonal metabolism. One of its physiological functions is the production of retinoic acid, which regulates the expression of multiple genes that in turn control vital processes such as cell proliferation and differentiation. Numerous studies have shown that altered enzyme activity in ALDH1A3 is associated with diseases such as cancer, diabetes, and obesity.
ALDH1A3 is also a biomarker of cancer stem cells, also known as tumor-initiating cells. It is believed that these cells are largely responsible for the emergence of resistance to chemotherapy, as well as relapse and metastasis in cancer. In recent years, ALDH1A3 has become a new therapeutic target and, in fact, its silencing restores the sensitivity of tumor cells to chemotherapeutic agents.
Thus, the blockade of ALDH1A3 activity, through pharmacological inhibitors (molecules that bind the enzyme decreasing its activity) co-administered with conventional anticancer agents, could help to improve the therapeutic response. For this reason, the study of the structure-function relationships in ALDH1A3 may open the door to the rational design of new therapeutic agents.
In the manuscript published in the journal Communications Biology, the research team from the Autonomous University of Barcelona and the ALBA Synchrotron describes for the first time the molecular structure of three configurations of ALDH1A3, obtained by X-ray crystallography in the . Thanks to the synchrotron light, these new structures make possible to precisely know the position of the atoms of the enzyme and how it binds to its cofactor NAD or ATP, also known as "the energy currency of the cell".
The study highlights the discovery of the binding of the ATP molecule to a member of the ALDH superfamily. It was also observed that ATP acts as a reversible inhibitor of ALDH1A3, thus ATP could modulate the enzymatic activity upon changing the energy state of the cell. On the other hand, it is known that the intracellular levels of ATP are higher in tumor cells and, especially in those that have become resistant to drugs, than in normal or drug-sensitive cells of the same cellular origin. In addition, ALDH1A3 is more abundant in cancer cells, which can promote ATP production. In contrast, the inhibition of the enzyme in cancer cells leads to a significant decrease in glucose consumption and a reduction in lactate and ATP production. This modulation of ALDH1A3 activity by ATP may also be relevant in other conditions, such as diabetes and obesity.
In summary, the results of this work allow scientist to speculate that the energy state of the cell is associated with ALDH1A3 and its potential role in metabolic reprogramming caused by diseases such as cancer, diabetes, and obesity. In addition, the availability of new high-resolution three-dimensional structures supports the rational design of inhibitors against ALDH1A3 and may drive the discovery of new drugs.
Text adapted from Universitat Autònoma de Barcelona
With the collaboration of Fundación Española para la Ciencia y la Tecnología. The ALBA Synchrotron is part of the UCCs of the FECYT and has received support through the FCT-20-15798 project.