ALBA Synchrotron
Researchers from ALBA, UPC and UNSAM have studied the chemical state of cobalt in three different cobalt-based catalysts during the ethanol steam reforming (ESR) reaction. Complementary analyses have been carried out with synchrotron light at CLÆSS and CIRCE beamlines. Cobalt-based catalysts are a promising alternative for hydrogen production, which could be used in vehicles or industrial applications in the future.
Cerdanyola del Vallès, 21st December 2018.
Hydrogen represents a clean and sustainable way of energy production. Despite it's one of the most common elements in the universe, its production is not easy and finds some environmental and economic difficulties. Hydrogen generation from current fossil fuels emits huge quantities of greenhouse gas, carbon dioxide. Besides, some systems to obtain hydrogen are still very expensive and difficult to extent to industrial scale.
The ethanol steam reforming (ESR) reaction is a safe and controlled process for generating hydrogen on board. However, this chemical reaction is complex and can result in the deactivation of the catalyst by carbon deposition. Preliminary ex situ studies have pointed out the role of metallic cobalt (Co) as nucleation point for the growth of carbon but still there is controversy in this respect.
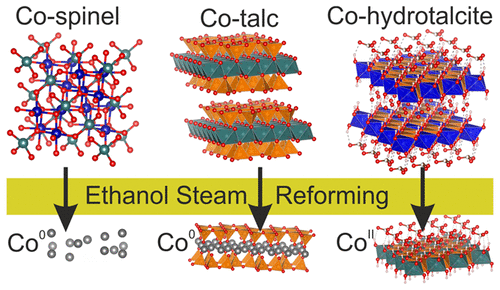
Now, researchers from the ALBA Synchrotron, the Institute of Energy Technologies from the Universitat Politècnica de Catalunya (UPC) and the Escuela de Ciencia y Tecnología from the Universidad Nacional de San Martín (UNSAM, Argentina) have analysed with synchrotron light the behaviour of three types of cobalt-based catalysts under operando conditions: Co-spinel, Co-talc and Co-hydrotalcite.
Previous catalytic reactivity tests performed by the group of Prof. J. Llorca (UPC), one of the authors of this scientific contribution, showed that the carbon deposition rate for Co-spinel and Co-talc was much higher than for the Co-hydrotalcite. Besides, the latter showed prolonged high activity for H2 generation during more than 300h. Now, they have demonstrated that the Co-hydrotalcite catalyst do not show metallic cobalt traces under the ESR reaction conditions, and this is what ensures a long-term stable operation of the catalyst since the carbon deposition then is not significant. These results are key to understand why deactivation of other cobalt-based catalysts occurs since confirm that metallic Co is the main responsible for the carbon growth, as suggested by previous ex situ studies.
"For the Co-hydrotalcite catalyst we did not observe the formation of metallic cobalt under reaction conditions while for the other two types of cobalt catalysts studied, Co-spinel and Co-talc, we have observed that the cobalt is totally or partially reduced to its metallic state in situ under the ESR conditions. This confirms that metallic Co is the responsible for the nucleation and deposition of carbon that ends up deactivating most of the cobalt-based catalysts during the ESR reaction", says Carlos Escudero, researcher of the ALBA Synchrotron.
To carry out this study, researchers have been using two complementary beamlines of the ALBA Synchrotron. They have performed in situ X-ray absorption measurements at in order to analyse the chemical state of cobalt obtaining more bulk-related information and they have also used the , much more surface sensitive, to analyse the same samples. Both experiments were done in situ, simulating as close as possible the real reaction conditions for the ESR reaction.
"This research paves the way for producing more effective catalysts for hydrogen production. Finding better catalysts will definitely boost hydrogen generation and will therefore minimize environmental pollution", says Carlos Escudero.
