ALBA Synchrotron
For the first time, it has been demonstrated that oxygen functionalities (epoxy, carbonyl, and lactone) on the carbon electrode surface do not affect the kinetics of one-electron oxygen reduction in aprotic media, but enhance both the rate of electrochemical lithium peroxide (Li2O2) formation and carbon degradation in Li+-containing electrolytes. The research has included analysis using synchrotron light at Elettra and ALBA facilities.
The electrochemical reduction of oxygen plays a significant role in many critical applications such as gas sensors, hydrogen peroxide electrosynthesis, and electrochemical energy storage. Oxygen reduction reaction (ORR) drives the operation of fuel cells and metal-air batteries. The latter potentially can provide the highest specific energy among energy storage devices.
To increase the ORR efficiency, a catalyst immobilized on (or mixed with) conductive support is introduced to the positive electrode composition. Usually, porous sp2-carbon materials, like graphene, serve as such supporting materials. Its electronic configuration (sp2) provides the sufficient electric conductivity to the positive electrode. Nevertheless, ORR proceeds too slowly on the neat surface of ideal sp2-carbon in the absence of a catalyst.
The role of graphene imperfections (vacancies, impurity atoms, and functional groups) on catalyzing ORR (mainly in aqueous media) has been under intense investigation during the last decades. However, little is known about the effect of oxygen functionalization of carbon on ORR in aprotic media (lacking the acidic protons). The interest in this process, especially in the presence of metal ions in the electrolyte, is relevant for various aprotic metal-oxygen batteries (lithium, sodium, magnesium, etc.) which are now considered as the most promising electrochemical power sources due to their outstanding theoretical performance. For such devices carbon electrodes are highly attractive due to their light weight and low cost, and the effect of carbon surface chemistry on the processes occurring upon battery operation is of great importance.
The present research shows for the first time that oxygenation of carbon electrode surface does not affect the rate of one-electron oxygen reduction in aprotic media. At the same time, in Li+-containing electrolytes, oxygen groups enhance both the rate of electrochemical Li2O2 formation and carbon electrode degradation due to faster oxidation by lithium superoxide (LiO2) intermediate yielding carbonate species as a product.
The research is led by scientists from Lomonosov Moscow State University and the Semenov Institute of Chemical Physics, in collaboration with Friedrich-Alexander-Universität Erlangen-Nürnberg, IFW Dresden, Saint Petersburg State University, Donostia International Physics Center and Massachusetts Institute of Technology.
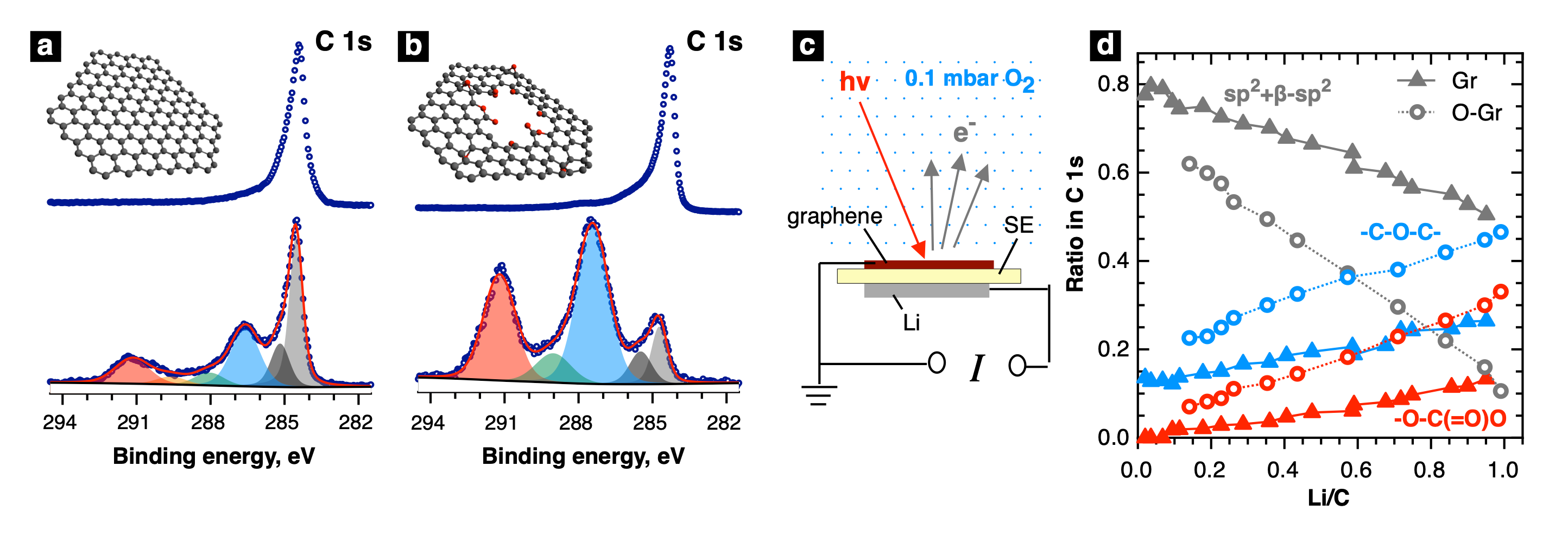
Researchers have used two model carbon materials: i) single-layer graphene grown either on copper foil or the surface of an iridium single crystal and ii) highly oriented pyrolytic graphite (HOPG). Both materials were oxidized to various degrees by atomic oxygen generated in a plasma source, or ozone under UV irradiation. The nature and quantity of oxygen-containing groups appearing upon the oxygenation were determined by XPS using C 1s spectra. The effect of oxygen functionalization on the 1st and 2nd electron transfer rate, as well as on carbon degradation rate, was evaluated by cycling voltammetry technique.
“We observed experimentally that epoxy and carboxylic functionalities do not affect the first electron transfer step from the carbon electrode to an oxygen molecule. In contrast, further electrochemical reduction to Li2O2 is catalyzed by the epoxy groups.” commented Lada Yashina, principal investigator of the study. “However, these groups also promote the carbon oxidation by LiO2 intermediate, which was analyzed with XPS studies performed under real working (operando) conditions”, added.
This promotion of carbonate formation by oxygen functional groups was finally confirmed by the direct spectral observation of graphene degradation, using operando XPS at NAPP endstation of CIRCE beamline, at ALBA, during the discharge of the model solid-state Li-O2 electrochemical cell. Two graphene samples were transferred from copper foil onto Li+-conductive solid electrolyte membranes; one of the samples was oxidized by UV-treatment in oxygen atmosphere. The cells were assembled on the sample holder in the Ar-filled glove box and transferred inside the analytical chamber of the spectrometer without air exposure. The cells were discharged inside the near ambient pressure XPS chamber in 0.1 mbar of O2 while acquiring C 1s, O 1s, and Li 1s core-level spectra. The growth of lithium and oxygen signals indicated the formation of discharge product; in C 1s spectra was observed an occurrence of two components assigned to carbonate species and ether-like functionalities. After comparing the C 1s component fractions for oxidized and non-oxidized graphene electrodes at the same discharge depth, it was found that more carbonate, as well as more ether-like groups, is formed on oxidized graphene, thus indicating in conclusion, a higher degradation rate.