ALBA Synchrotron
A research team has developed a synthesis procedure to modulate the exact interaction between platinum (Pt) and tin (Sn) in subnanometric Pt clusters confined in MFI (Mobil-type five) zeolite to achieve optimal catalytic performances. The catalyst showed an initial propane conversion of ~20% and after 70 h the propane conversion decreased very slowly to ~17%, demonstrating the outstanding performance of this new catalyst. In-situ X-ray absorption spectroscopy (XAS) experiments were performed in the CLÆSS beamline at the ALBA Synchrotron.
Platinum-based materials are widely used for reforming, hydrogenation and dehydrogenation catalysts in numerous industrial processesdue to their excellent properties for the activation of carbon-hydrogen (C–H) bonds while limiting the extent of C–C bond cleavage through hydrogenolysis.
Researchers from the Instituto de Tecnología Química (Valencia, Spain) in collaboration with the ALBA Synchrotron, the Universidad de Cádiz (Andalusia, Spain), and ExxonMobil Research and Engineering (USA); have developed a synthesis procedure to modulate the exact interaction between platinum (Pt) and tin (Sn) upon introduction of partially reduced Sn species in subnanometric Pt clusters (0.5–0.6 nm), confined in purely siliceous MFI (Mobil-type five) zeolite to achieve optimal catalytic performances. The platinum–tin (PtSn) clusters are stable in hydrogen (H2) for more than six hours even at high temperatures (600 °C).
The introduction of a second metal into nanoparticulate metal catalysts can modulate the electronic structure of pristine metals and further influence their catalytic behavior. By tuning the procedure for preparing the bimetallic nanoparticles or by post-synthetic treatments, the spatial distribution of the two elements as well as their chemical states can be modified, thus, their reactivity.
The as-prepared bimetallic PtSn catalyst showed an order-of-magnitude-lower deactivation rate in the propane dehydrogenation while maintaining high catalytic activity.
In this study, the researchers tested the catalytic performances of PtSn catalyst for propane dehydrogenation reaction using conditions similar to those used in industrial processes. The catalyst showed an initial propane conversion of ~20% at 550 °C and after 70 h the propane conversion decreased very slowly from ~20 to~17%, demonstrating the outstanding performance of this new catalyst.
A comprehensive study through a combination of techniques
Several characterization techniques were used for a deep study of these materials, including X-ray absorption spectroscopy (XAS), quasi in situ transmission electron microscopy (TEM), high-resolution high-angle annular dark-field scanning transmission electron microscopy (HR-HAADF-STEM) and carbon monoxide (CO) infrared (CO-IR) spectroscopy
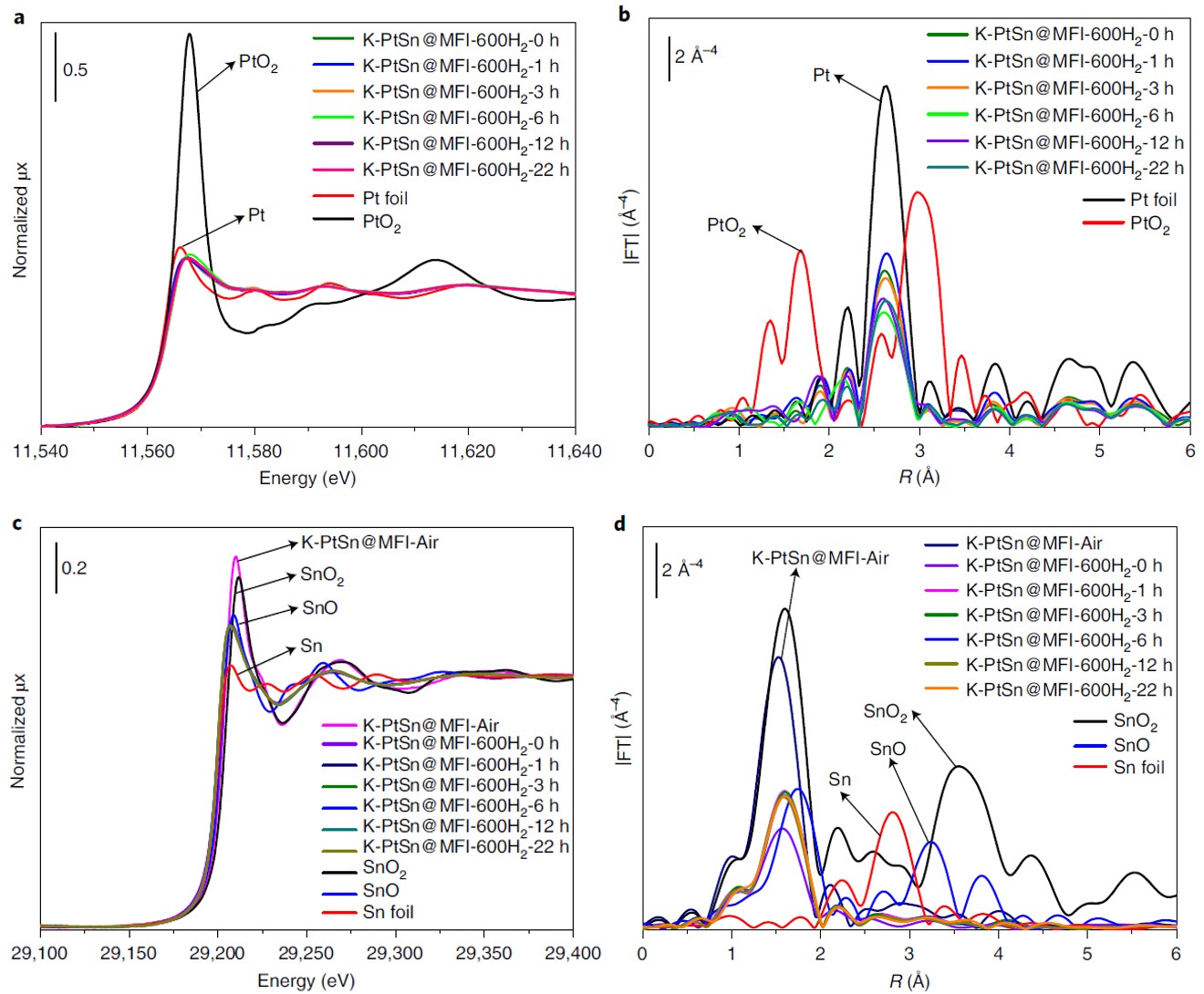
In-situ XAS at Pt LIII-edge and Sn K-edge was performed during H2 reduction at 600° in the CLÆSS beamline at the ALBA Synchrotron to study the evolution of chemical state and local structure of Pt and Sn. Regardless of the reduction time, the Pt species were completely reduced to metallic Pt at 600 °C. In the pristine sample, Sn is present as atomically dispersed Sn(IV) species in the MFI zeolite. During H2 reduction, oxygen in the unreduced sample reacts with H2 to form SnOx with an average oxidation state close to Sn(II). Neither the Pt LIII-edge nor the Sn K-edge EXAFS spectra showed evidence of any kind of bimetallic interactions, thus excluding the formation of two metal alloys, in contrast with the results reported in the literature.
The formation of interactions between Pt clusters and partially reduced Sn species during the H2 treatment was also confirmed by CO infrared spectroscopy.
Quasi in situ TEM experiments revealed well-distributed subnanometric Pt clusters in the sinusoidal channels of porous zeolite. In order to obtain a semi-quantitative estimation of the spatial distribution of subnanometric Pt and Sn, HR-HAADF-STEM images were analyzed by a modified K-means clustering method. As the reduction time increases, the percentage of bimetallic PtSn clusters in the sample also increases, reaching >40% after exposure to H2 during 16 or 22 h.
With the collaboration of Fundación Española para la Ciencia y la Tecnología. The ALBA Synchrotron is part of the of the of UCC network from the FECYT and has received support through the FCT-20-15798 project.