ALBA Synchrotron
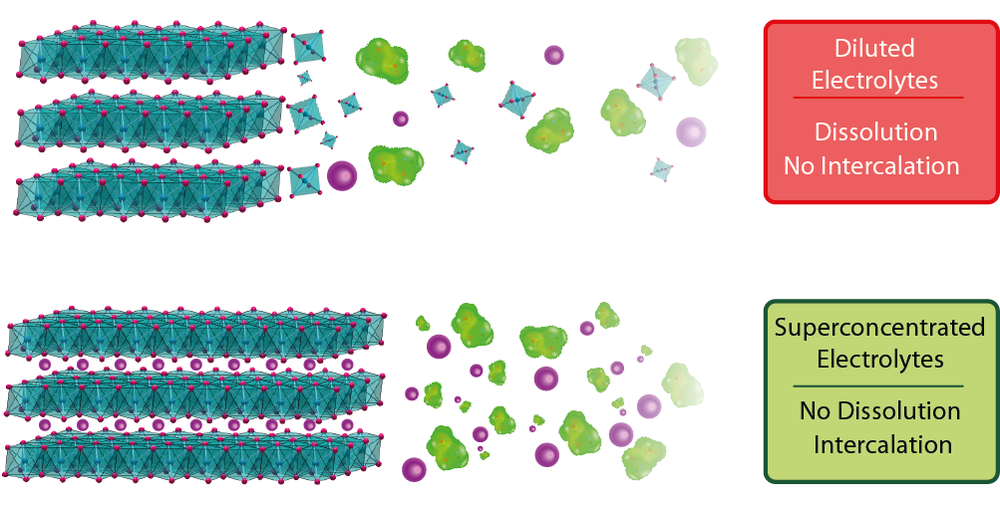
A new work published in Nature Materials demonstrates that a novel class of materials, namely transition metal halides, can be now employed as Li-ion batteries electrodes by using superconcentrated electrolytes instead of regular diluted electrolytes in which they dissolve. The study is led by the Collège de France and has used the MSPD beamline of ALBA to monitor the evolution of the metal halides structures upon cycling in a battery, which was crucial for the study.
It is not a surprise that the research field of batteries is gaining a considerable attention in the last years and huge efforts are being made to understand and design batteries with better performances. Many of our current personal electronic devices (laptops, tablets, cell phones, etc.) and also electric cars rely on the use of lithium-ion batteries. So, the race for achieving batteries that can be charged in less time or that can store more energy is already started.
Born in the early 1990s, lithium-ion batteries are charged and discharged when lithium ions travel back and forth between the negative (anode) and positive (cathode) electrode inside the battery. The cathode material is one of the most important components to determine the properties of the battery.
Till now, materials such as oxides, sulfides or polyanionic compounds were the cathodes of choice used in Li-ion batteries owing to their good stability in classical diluted organic electrolytes. “The finding of our study opens the route to a novel intercalation chemistry relying on transition metal halides so far unexplored owing to their relative solubility”, comments Nicolas Dubouis, scientist at the Collège de France.
The research team has proved that transition metal halides can be employed as Li-ion batteries electrodes by using superconcentrated electrolytes instead of regular diluted electrolytes in which they dissolve.
While the materials presented in the Nature Materials paper are a proof of concept with limited cycling performances, the halide chemistry is vast and high energy density halides materials can now be envisioned as Li-ion batteries cathode either as intercalation or conversion compounds.
The study has been led by scientists from the Collège of France, the Sorbonne Université, the Réseau sur le Stockage Electrochimique de l’Energie (RS2E) and the CNRS-CEMHTI in collaboration with the University of Sydney and the Australian Nuclear Science and Technology Organisation.
For the analysis, experiments at ALBA Synchrotron have been performed using operando X-ray diffraction on MSPD beamline to monitor the evolution of the vanadium halides structures upon cycling in a battery. To do so, researchers assembled lithium batteries using the materials of interest as electrodes in “coin cell” devices (similar to the small commercial batteries). These specific cells were equipped with a transparent window that allows transmission of the beam light across the entire cell and probe the material structure in real-time during the battery operation.
"The intensity of the synchrotron light used in the diffraction experiments was crucial to acquire high-resolution data in a very short time and help characterizing very precisely the mechanism for the electrode material reaction upon cycling", points out Dubouis. These synchrotron experiments confirmed that vanadium halides can be used as hosts to reversibly intercalate lithium-ions when combined with superconcentrated electrolytes.
Moreover, the new findings open a new synthetic route for designing materials with tunable physical properties as illustrated in a follow-up article, just accepted in Physical Review B, with François Fauth (beamline responsible of MSPD beamline) as co-author, describing the magnetic properties of lithiated vanadium halides.
With the collaboration of Fundación Española para la Ciencia y la Tecnología. The ALBA Synchrotron is part of the of the UCC of the FECYT and has received support through the FCT-20-15798 project.
