ALBA Synchrotron
An international research team from Germany and Spain has shown that the combination of ruthenium (Ru) and rhodium (Rh) single-atom catalysts stabilized at the surface of cerium dioxide (CeO2) creates a synergetic effect enabling a highly selective olefin isomerization-hydrosilylation tandem reaction. The process is highly relevant from an industrial point of view considering that complex mixtures of olefins are used as starting materials for plastics, alongside a straightforward catalyst recycling and reuse.
Isolated metal atoms, stabilized on the surface of oxide carriers, attract great attention as active sites in heterogeneous catalysis. These single-atom catalysts (SACs) not only maximize the utilization efficiency of expensive metals of rare resources but also have a great potential to significantly improve the selectivity of the targeted catalytic reactions and lower the operation costs.
A relevant area where SACs can have a profound impact is tandem catalysis, that is, the integration of two catalysts in a single pot to achieve sequential transformations in a direct manner. These one-pot processes avoid the time, labor and yield losses associated with the isolation and purification of intermediates in multistep sequences.
A research team, formed by scientists from the Max-Planck-Institut Kohlenforschung, the Max-Planck-Institut Chemische Energiekonversion and the Karlsruhe Institute of Technology (Germany), and the Laboratorio de Microscopias Avanzadas and the ALBA Synchrotron (Spain), have shown that the combination of Ru and Rh Single-Atom Catalysts (SACs) stabilized at the surface of CeO2 create a synergetic effect enabling a highly selective olefin isomerization-hydrosilylation tandem process, hitherto restricted to molecular catalysts in solution. Terminal organosilane compounds, which are of utmost technological significance in areas such as functional coatings, polymer cross-linking and the manufacture of a wide variety of composite materials, can be thus produced with similarly high regio-selectivities from both terminal and internal olefin substrates, as well as mixtures thereof.
Present studies provide a better understanding of the single-pot cooperation in Ru1/Rh1 single-atom metal catalysts, that allows the production of terminal organosilane compounds with high regio-selectivity (>95%). The process is highly relevant from an industrial point of view considering that complex mixtures of terminal and internal olefins are used as starting materials for synthetizing plastics, alongside a straightforward catalyst recycling and reuse.
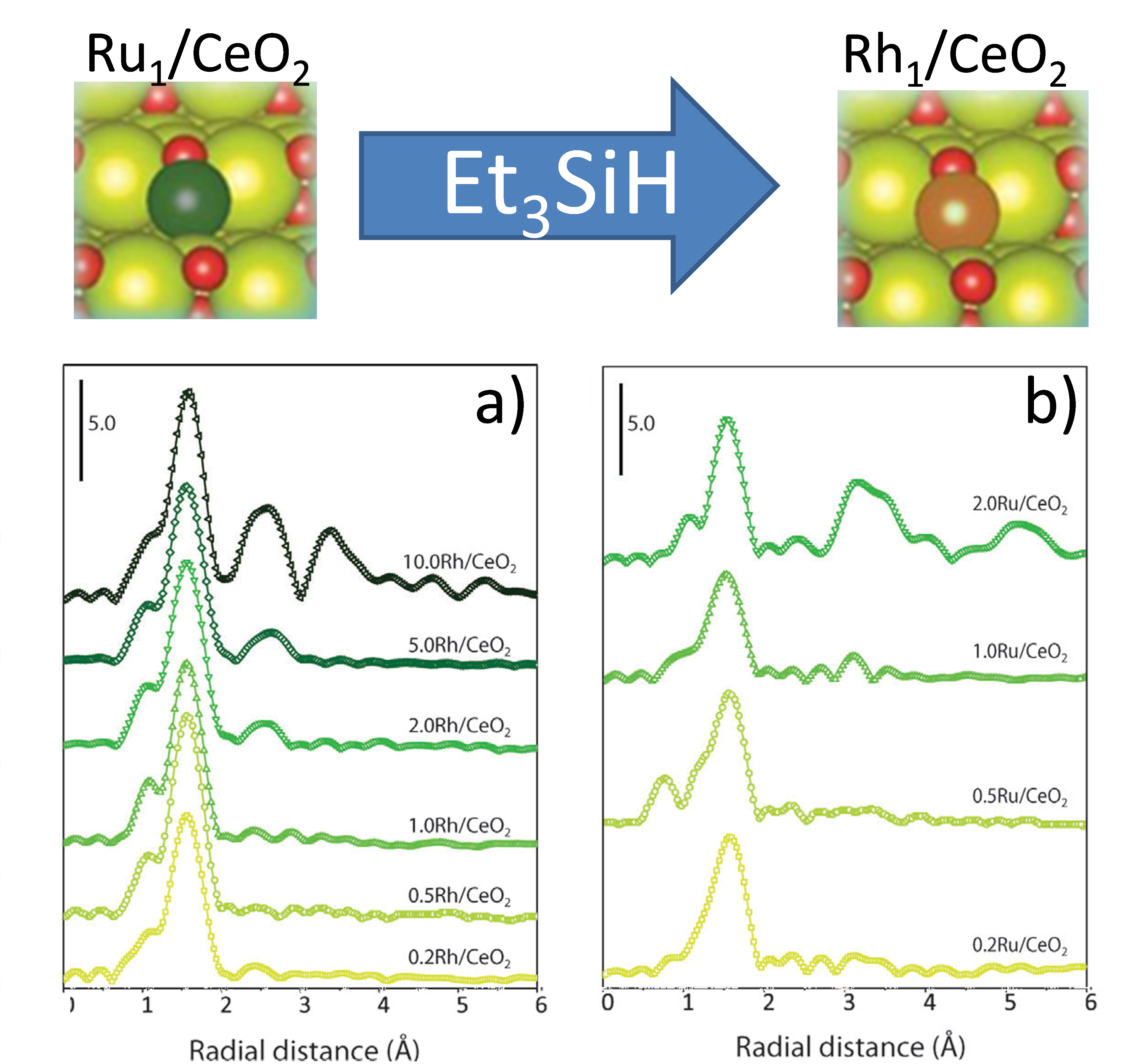
To achieve the characterization of this tandem catalytic reaction, the authors combine complementary experimental techniques - like multi-edges X ray absorption spectroscopy (XAS), X-ray photoelectron spectroscopy (XPS), Fourier Transform Infrared spectroscopy (FTIR) and Transmission Electron Microscopy (TEM) - and theoretical First-principles DFT calculations in order to correlate the chemical selectivity with differences in the binding strength of the olefin substrate to the monoatomic metal centers.
The authors used TEM, XRD (X-ray diffraction) and FTIR to get insights into the atomicity and spatial distribution and selectivity of the metal species as preliminary characterization of the materials. In particular TEM results indicate the absence of aggregation of metal species, while the analysis of infrared spectra has permitted to define the saturation levels of the SACs catalysts. Ex situ EXAFS (extended X-ray absorption fine structure) measurements, at the CLAESS beamline of the ALBA Synchrotron, allowed to determine the atomicity and coordination environment of metal species (Platinum (Pt), Ru, and Rh) during the reaction. The estimation of the coordination numbers has discarded the presence of Rh dimerization or oligomerization prior and after catalysis induction.
Results demonstrate that the single-pot cooperation of CeO2-supported Ru and Rh-based single-atom catalysts realizes a tandem catalytic process that is capable of reconciling the reaction specificity typical of molecular catalysts with the stability and technically simple recycling inherent to solid catalysts. The technological significance of this approach is herein demonstrated with the direct and selective conversion of complex mixtures of olefin regio-isomers to terminal organosilanes.
Beyond this showcase process, obtained results provide a blueprint to exploit a new dimension of oxide-supported single-atom metal catalysts, in the area of tandem catalysis, which can decisively contribute to realizing their potential as a bridge between the realms of homogeneous and heterogeneous catalysis.
With the collaboration of Fundación Española para la Ciencia y la Tecnología. The ALBA Synchrotron is part of the of the of UCC network from the FECYT and has received support through the FCT-20-15798 project.