ALBA Synchrotron
A study from several Italian institutions and the ALBA Synchrotron suggest crystalline calcium carbonate as a precursor of hydroxyapatite in the process of bone formation. Since hydroxyapatite is a mineral constituting 70% of the mass of bone, these findings may have potential applications in the development of new therapeutic approaches in bone cancer. Thanks to the MISTRAL beamline at ALBA, researchers were able to create a 3D tomogram of human cells and visualize calcium depositions inside them.
Stem cells are “non-specialized” cells that can differentiate (transform) into a specific type of cell with a specific function. To become bone cells, stem cells need to “learn” how to take calcium to form the bones. This is related to biomineralization, a process by which living organisms produce minerals, often to harden or stiffen existing tissues. Calcium is known to be found in bones in the form of hydroxyapatite, which is a naturally occurring mineral form of calcium apatite and represents approximately 70% of the mass of bones.
In human cells, biomineralization culminates with the formation of hydroxyapatite, but the mechanism that explains the origination inside the cell and the propagation of the mineral in the extracellular matrix remains largely unexplained, and its characterization is highly controversial, especially in humans.
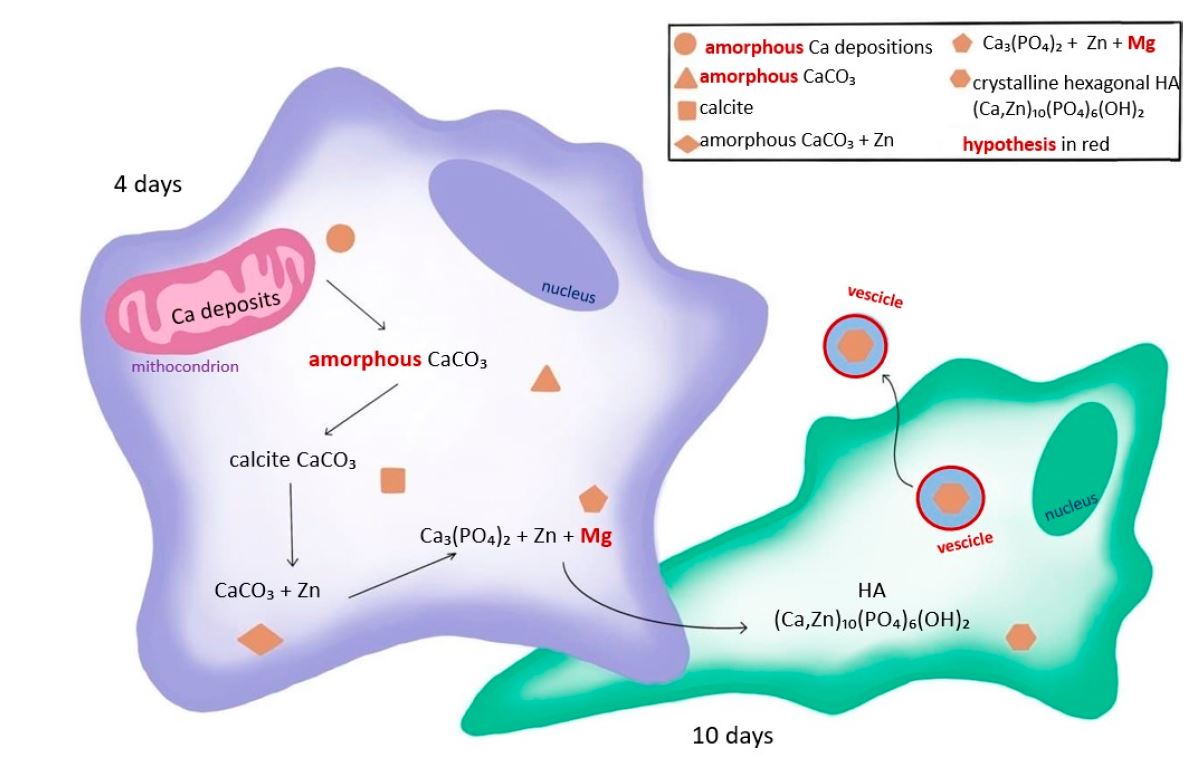
An interdisciplinary research team, formed by several Italian institutions and the ALBA Synchrotron, used synchrotron-based techniques to characterize the contents of calcium depositions in human stem cells induced to differentiate towards bone cells (osteoblasts). They compared the results for cells at 4 and 10 days after the osteoblastic induction.
Results showed that a crystalline form of calcium carbonate, probably calcite, is present in stem cells at 4 days, while hydroxyapatite crystals and no calcite depositions were found in stem cells at 10 days. This suggests that calcite is one of the precursors in the biomineralization process, meaning that bone formation does not start from an amorphous calcium phosphate compound, as previously thought, but from calcium carbonate.
These findings have potential applications in the development of new therapeutic approaches in bone cancer (osteosarcoma), exploiting differentiating agents.
These substances cause cells to change from an immature form to a mature form. Malignant tumor cells tend to assume a less specialized, stem cell-like dedifferentiated state, so therapies based on differentiating agents may help cancer cells become more like normal cells and grow and spread more slowly.
The team in charge of the study is interdisciplinary involving physicists, informatics, chemists, biochemists, biologists and anatomists. The collaborators are the University of Bologna, the University of Milan, the National Institute of Biostructures and Biosystems (Rome), the Chemical Center S.r.l (Bologna) and the MISTRAL beamline in the ALBA Synchrotron (Barcelona).
X-ray tomography: a 3D map of the cells

Figure. (d) Color-coded 3D rendering of the nucleus and dense structures of bone mesenchymal stem cell 4 days after osteoblastic induction. Dense structures voxels were selected automatically using a threshold. After spectral analysis, only some of these dense structures show Calcium content. These Calcium-rich voxels are reported in (e). Scale bar is 2 μm. / Images based on cryo-X-ray absorption near edge structure microscopy.
Researchers used cryo-soft-X-ray tomography (cryoSXT) and cryo-X-ray absorption near edge structure microscopy (cryoXANES) techniques at the MISTRAL beamline in the ALBA Synchrotron. MISTRAL allows a 3D view of cells in their natural state. Thanks to this cutting-edge technique, which is only available in three other synchrotron light facilities around the world, biomedical researchers can perform a tomogram of a cell with a thousand times better resolution than a conventional CT scan.
This specific study aims at localizing and characterizing the crystalline phase and the calcium concentration in the calcium-rich depositions at the early phase of biomineralization in the stem cells studied. The combination of these techniques permits to study biomineralization at the intracellular level in frozen-hydrated whole cells at native state conditions and with a spatial resolution of few tens of nanometers.
CryoSXT consists of recording 2D soft X-ray transmission projections on a detector changing the sample orientation with respect to the incident synchrotron light. Then, 3D maps of the sample are generated by applying a reconstruction algorithm.
By scanning the energy across the edge of interest, cryoXANES provides pixel-by-pixel absorption spectra and can be exploited to determine the 2D chemical state of both light and heavy elements in the sample.
With the collaboration of Fundación Española para la Ciencia y la Tecnología. The ALBA Synchrotron is part of the UCCs of FECYT and has received support through the FCT-20-15798 project.