ALBA Synchrotron
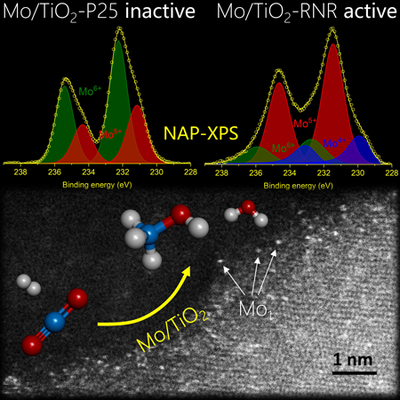
They proved that a system based on molybdenum-on-titania does efficiently catalyze this reaction and represents a promising alternative to current industrial catalysts. This helps eliminating carbon dioxide and producing methanol, which can serve as a "green fuel" or as building blocks for the production of plastics, pharmaceuticals, textiles, etc. The team carried out experiments at the CIRCE-NAPP beamline of the ALBA Synchrotron to characterize the structure of the catalyst.
Due to environmental concerns, and following the European Commission's climate strategies and targets, the reduction of greenhouse gas emissions has become mandatory. In this context, the most promising strategy for the valorization of carbon dioxide (CO2) consists of the conversion of CO2 to methanol by hydrogenation (reaction with hydrogen – H2). This process has the double advantage of eliminating CO2 (present in the atmosphere or in industrial effluents) and producing a valuable compound, methanol, which can serve as a fuel (e.g. in car fuel cells) or as building blocks for the production of plastics, pharmaceuticals, textiles, etc.
Hydrogenation is a process that takes place in the presence of a catalyst, but the one that is mostly used for the industrial production of methanol presents some disadvantages that lead to its deactivation during the reaction. Hence, it is desirable to find alternative catalysts with enhanced performances regarding stability, activity and selectivity to methanol.
Researchers have shown in a recent study that the system molybdenum-on-titania (Mo/TiO2), which was not known until now to be a CO2 hydrogenation catalyst, does in fact catalyze CO2 hydrogenation toward methanol. In addition, they found out that its activity and selectivity strongly depend on the type of titania (atomic structure and crystallite shape) and the molybdenum coverage employed. The optimal system consists of a few weight percentage of molybdenum "ultradispersed" over rutile titania nanorods.
After the preparation of the catalyst, the research team was able to characterize its structure with several analytical techniques, including Near-Ambient Pressure X-ray Photoelectron Spectroscopy (NAP-XPS) preformed at the , and advanced electron microscopy. These experiments allowed to identify reducible Mo-Ti oxide surface species as the active catalytic sites (where the reaction takes place), in presence of the gaseous reactants (CO2, H2). The most active catalyst for methanol production involves isolated molybdenum cations with lower oxidation state, anchored on rutile titania nanorods (as seen in the figure above).
This study is a collaboration between researchers from French and Spanish institutions: the Institut de Recherche sur la Catalyse et l'Environnement de Lyon (IRCELYON), the Institut de Physique et Chimie des Matériaux de Strasbourg, Laboratoire Hubert Curien, the ALBA Synchrotron and the Institute of Energy Technologies (UPC). This work was financially supported by French ANR, UltraCat project.
With the collaboration of Fundación Española para la Ciencia y la Tecnología. The ALBA Synchrotron is part of the of the Unidades de Cultura Científica y de la Innovación (UCC+i) of the FECYT and has received support through the FCT-20-15798 project.
