ALBA Synchrotron
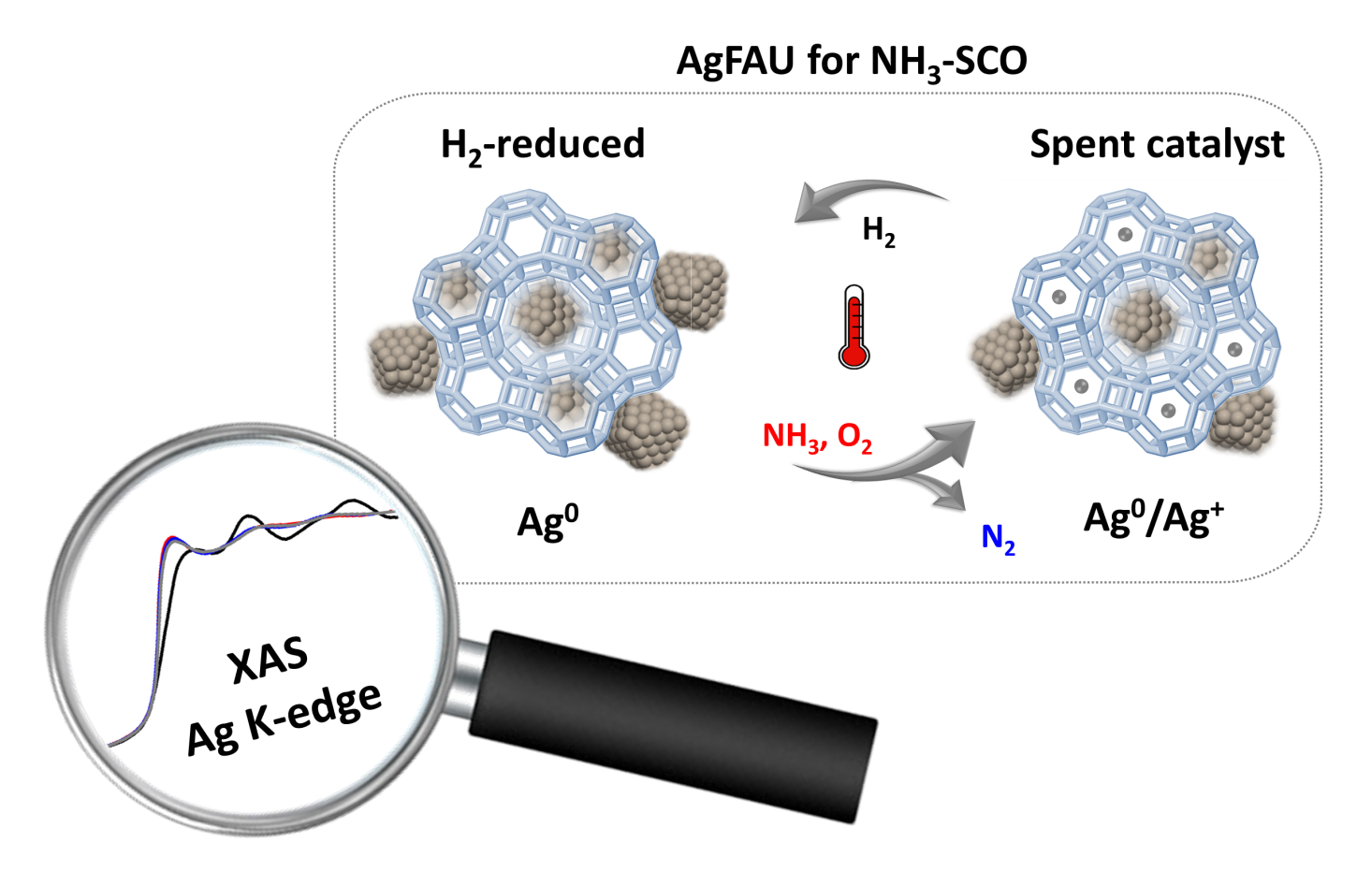
Researchers from the ITQ-UPV-CSIC, in collaboration with ALBA, have explored the use of silver nanoparticles as catalysts for the selective catalytic oxidation of ammonia, one of the main atmospheric pollutants. Thanks to the CLÆSS beamline at ALBA, researchers proved that the active catalyst for the reaction of ammonia to nitrogen and water is metallic silver, instead of silver cations.
These findings will contribute to developing new methods for the elimination of ammonia released to the atmosphere in industry and in diesel vehicles.
Ammonia is one of the main atmospheric pollutants and damages both the human health and the environment. Most ammonia emissions come from fertilizers used in agriculture, but it is also released to the atmosphere in biomass burning, fuel combustion and industrial processes, in which unreacted ammonia escapes into the atmosphere in the exhaust gases.
In the last years, more strict environmental regulations have been intensified with the aim to develop new methods for the elimination of this pollutant. The most promising technology is the selective catalytic oxidation of ammonia to nitrogen and water.
In a recent publication, researchers from the Instituto de Tecnología Química, Universitat Politècnica de València - Consejo Superior de Investigaciones Científicas (UPV-CSIC), in collaboration with ALBA, have explored the use of silver-containing zeolites (microporous aluminosilicates) for the catalytic oxidation of ammonia. Results confirmed that the active site for the reaction is the silver found in the form of metallic nanoparticles at the external surface of the zeolite, whereas silver cations (Ag+) are practically non-active.
Furthermore, the experiment proved that silver nanoparticles present in the active catalyst were dispersed and oxidized to silver cations during the reaction. These findings will allow the scientific community to develop a method for removing ammonia released to the atmosphere in industry and in diesel vehicles.
The experiment, performed at the CLÆSS beamline in the ALBA Synchrotron, allowed to study the catalysts under reaction conditions. The researchers recorded several X-ray absorption spectra (XAS) while submitting the samples to the reactive atmosphere (ammonia and oxygen) at increasing temperatures. Results showed that the silver nanoparticles formed before the reaction were dramatically modified under reaction conditions, being most of them dispersed and resulting in small clusters and cations Ag+.
The experiment carried out at ALBA by Christian W. Lopes, led by Teresa Blasco and other members of Fernando Rey’s group from ITQ-UPV, is part of a large ongoing project that involves other international facilities, such as the Institute Laue-Langevin in Grenoble, the Jagiellonian University in Poland as well as the Cepsa’s Research Center, which is one of the major refining and chemical holding in Spain.
With the collaboration of Fundación Española para la Ciencia y la Tecnología. The ALBA Synchrotron
is part of the of the Unidades de Cultura Científica y de la Innovación (UCC+i) of the FECYT and has received support through the FCT-20-15798 project.
