ALBA Synchrotron
Due to its chemical reactivity and mobility, sulfur is an essential component of the matter. However due to several experimental constraints related to the energy range of the sulfur absorption edge, direct studies at the sulfur K edge are still challenging. Thanks to its versatility, high photon flux and detection capabilities, CLÆSS provide easy access to the investigation of the electronic and structural properties around this light element in matter.
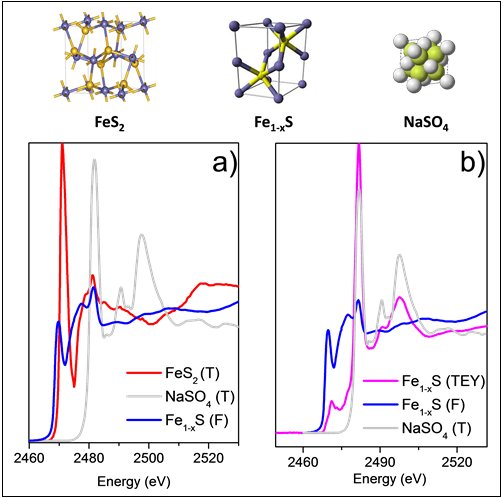
Sulfur has a particularly interesting X-ray absorption edge spectrum, with chemical shifts spanning more than 14 eV [1]. This permits to easily identify and quantify the presence of different sulfur species in the sample. For example in the figure we report spectra acquired at CLÆSS that enlighten the energy shift due to the different sulfur oxidation states. In panel a) are shown the spectra of iron pyrites (FeS2), iron pyrrhotite (Fe1-xS), and sodium sulfate (Na2SO4) measured in transmission (T) and fluorescence (F) modes, with integration time of about 5-15 minutes each one.
Apart from the already commonly used transmission and fluorescence detection modes, Total Electron Yield (TEY) has been recently successfully commissioned at CLÆSS. Differently from transmission and fluorescence, were photons are collected, in TEY the absorbed X-ray intensity is measured through the ejected photoelectrons which are proportional to the absorption. Taking into account that the main free path of electrons is limited to the nanometer scale, while photons of that energy penetrate more, around few microns, the TEY is, as a consequence, more surface sensitive. This explains the differences observed in the two spectra collected simultaneously on pyrrhotite and reported in panel b) of fig. The main peak in the TEY spectra is located around 2480 eV, exactly in correspondence with the main peak of the sodium sulfate (grey line), suggesting that the sample surface is oxidized.

Fig. Normalized sulfur K-edge absorption spectra collected in transmission and fluorescence mode (panel a) and in transmission, fluorescence and TEY mode (panel b) on selected references (FeS2, Fe1-xS, and Na2SO4).
CLÆSS beamline is dedicated to X-ray absorption and emission spectroscopies. It is installed on a wiggler source and covers an unusually wide energy range: from 2.4 to 63 keV. In addition to different collection modes, several sample environments are available (setups from liquid He to room temperature and from liquid nitrogen to 1000K, as well as in situ catalytic reactors) so that the beamline meets the needs of a very large community of researchers, from fundamental chemistry and physics, to catalysis, cultural heritage, environmental science, food and health science, pharmaceutical science, material science, electrochemistry, energy storage and related fields, etc.
References: [1] G.N. George et al., Jor. Am., Chem. Soc, 111, 3182 (1989); Ingrid J. Pickering et al., FEBS Letters 441 (1998) 11-14; Hideo Sekiyama et al., Bulletin of the Chemical Society of Japan Vol. 59 (1986) No. 2 P 575-579.