ALBA Synchrotron
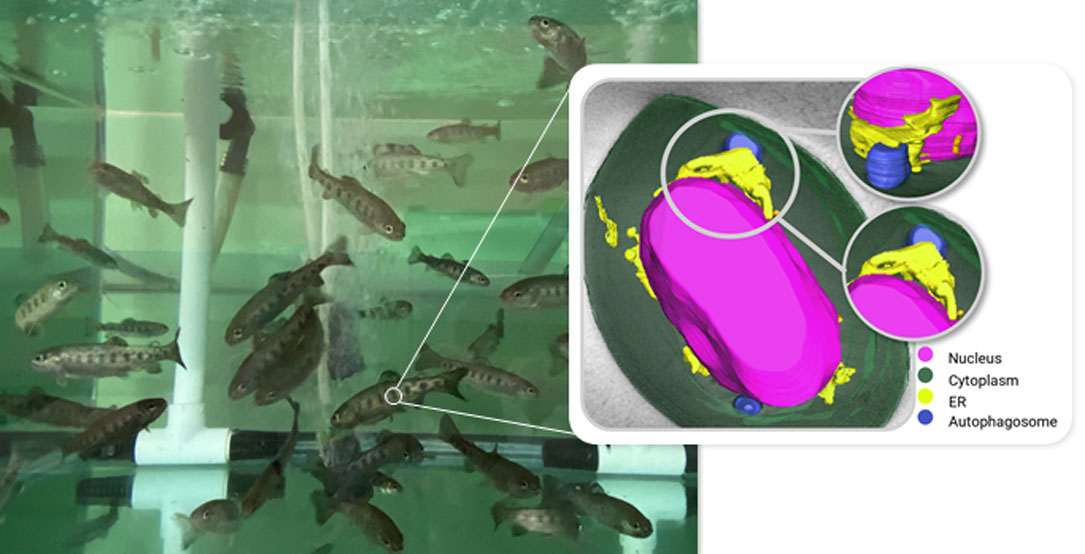
A collaborative study by the Institute for Research, Development, and Innovation in Health Biotechnology of Elche (IDiBE) and the ALBA Synchrotron has used cryo-soft X-ray tomography to investigate the viral response of rainbow trout red blood cells (RBCs) to the virus commonly known as 'fish Ebola'.
The research, published in Frontiers in Immunology, reveals significant cellular structural changes that could inform novel fish vaccine development, a critical need in aquaculture.
Fish nucleated red blood cells, also known as erythrocytes, play a crucial role in maintaining immune system balance in response to various stimuli, including viral attacks. Previous studies have shown that erythrocytes undergo intracellular changes - such as endoplasmic reticulum (ER) stress, autophagy, and antigen presentation - to prevent viral replication in response to viruses and DNA vaccines. A deeper understanding of this response could aid the development of new preventive treatments, particularly much-needed vaccines for the aquaculture industry.
To investigate these mechanisms, researchers from Institute for Research, Development and Innovation in Health Biotechnology of Elche (IDiBE) of the Miguel Hernández University (UMH) and the MISTRAL beamline at ALBA Synchrotron studied the response of rainbow trout erythrocytes when exposed to viral hemorrhagic septicemia virus (VHSV). This highly contagious virus severely affects various fish species, with mortality rates of up to 100%. For this reason, it has been commonly referred to as "fish Ebola."
To examine in detail the erythrocytes’ response, researchers employed advanced imaging techniques at the ALBA Synchrotron. Cryo-soft X-ray tomography (cryo-SXT) - available in five places all over the world, being one of them the MISTRAL beamline - is a novel approach that enables the visualization of 3D nanoscale structures in intact cryopreserved cells.
The research team purified rainbow trout erythrocytes and exposed them to VHSV. At the ALBA Synchrotron, they visualized the structural differences in infected cells, revealing that rainbow trout erythrocytes experience an increase of the endoplasmic reticulum volume and activate the endoplasmic reticulum stress process. Using molecular biology techniques, the team verified that this process activates the misfolded protein response (UPR). The UPR is an internal defence mechanism that cells trigger when faced with stress. Furthermore, by inhibiting reticulum stress, they discovered that the virus increased its replication, demonstrating that this process contributes to slowing the infection.
The results suggest that rainbow trout erythrocytes modulate endoplasmic reticulum stress as an antiviral control mechanism and open a new line of research to identify antiviral strategies targeting erythrocytes.
"Access to state-of-the-art facilities like the ALBA Synchrotron and the use of cryo-SXT have been essential in pushing the boundaries of our research. Thanks to these cutting-edge imaging techniques, we now have a clearer picture of how erythrocytes respond to viral infection and can focus on translating these findings into applied strategies. This knowledge opens the door to designing next-generation vaccines that not only boost the immune system but also optimize erythrocytes function as a highly strategic defence team. Our future research will explore how to fine-tune these responses to maximize antiviral protection", says researcher Maria Salvador Mira from IDiBE.
Members of the "Erythrocytes in Antiviral Immunology" group from the Institute for Research, Development and Innovation in Health Biotechnology of Elche (IDiBE) at the Miguel Hernández University (UMH) participated in this study, led by Dr. María del Mar Ortega-Villaizán Romo, and carried out by María Salvador-Mira, Dr. Verónica Chico Gras, Dr. Sara Puente-Marín, and Dr. Iván Nombela, in collaboration with Dr. Ana J. Perez-Berná, researcher at the MISTRAL beamline of the ALBA Synchrotron.

Workflow of the experimental process to study the changes in rainbow trout erythrocytes induced by viral hemorrhagic septicaemia virus (VHSV).