ALBA Synchrotron
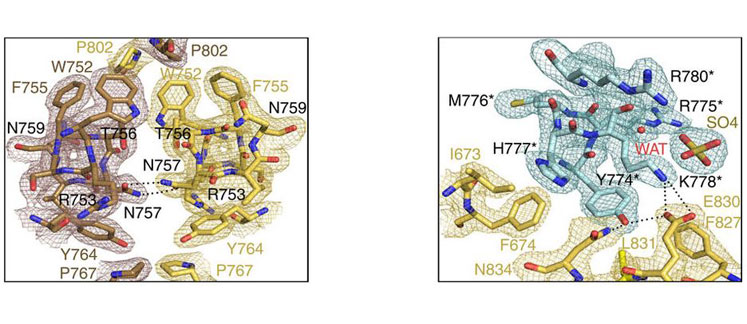
An international research lead by Institute of Biomedicine of the University of Barcelona (IBUB) and the Research Institute of the Hospital de la Santa Creu i Sant Pau (IIB Sant Pau) shows, for the first time, the three-dimensional structure of the homodimeric androgen receptor ligand-binding domain. In order to solve the 3D structure of the protein, X-ray diffraction analyses have been conducted at the XALOC beamline of the ALBA Synchrotron.
This work, published in Nature Communications, enables understanding the molecular bases of mutations detected in patients of prostate cancer and also in the ones with androgenic insensitivity syndrome (AIS), a disease caused by the mutations in the X chromosome that create a resistance to male hormones.
The human androgen receptor is a key protein in the regular development and activity of the prostate in response to male hormones such as testosterone. The deregulating activity of the receptor is directly related to the carcinogenesis of the prostate gland. Therefore, "the resolution of its three-dimensional structure is an essential key to diagnose and predict prostate cancer, as well as monitoring patient's resistance to medicine", says Eva Estébanez Perpiñá, professor from IBUB.
During the research, X-ray diffraction analyses were done at XALOC, the ALBA Synchrotron beamline devoted to protein crystallography. Thanks to synchrotron light, it was able to obtain with atomic resolution the structure of the protein.
The study has been protected by a patent application with both the University of Barcelona and the Research Institute Foundation of the Santa Creu and Sant Pau Hospital as co-owners.
The researchers have started exploring the design of a new generation of drugs in collaboration with pharmaceutical companies. The idea is that "the new drugs will block the physiopathological processes coming from the union of the natural hormones, instead of competing with their union –like current antiandrogens do", says Pablo Fuentes-Prior, scientist from IIB-Sant Pau.
It is worth-mentioning that Nature Reviews Urology has chosen this work as a highlight article because of its relevance in the field of urology and oncology.
The research, signed by Marta Nadal (IBUB) as its first co-author, counts with the participation of Marta Taulés, researcher of the Scientific and Technological Centers of the UB, and the team of Marta Vilaseca, researcher of the Institute for Research in Biomedicine, apart from researchers from the Univesity of Leuven (Belgium) and the Erasmus University Rotterdam (Netherlands). Also with Eva Estébanez-Perpiñá (IBUB) and Pablo Fuentes-Prior (IIB-Sant Pau) as corresponding authors.

Details of the electronic density map obtained by X-ray diffraction, and the outline of the aminoacid atoms (indicated with codes) which form the protein obtained from this map.
More information at:
http://www.ub.edu/web/ub/en/menu_eines/noticies/2017/02/008.html?
http://www.agenciasinc.es/Noticias/Identificada-una-diana-clave-para-tratar-el-cancer-de-prostata
http://www.nature.com/nrurol/journal/vaop/ncurrent/full/nrurol.2017.24.html