ALBA Synchrotron
Researchers have developed a new procedure for light olefins synthesis using Fischer-Tropsch reaction. They added tin and antimony to the iron catalysts, to increase their activity. This process, called promotion, resulted in a major increase in the reaction rate. Fischer-Tropsch synthesis provides an important opportunity for utilization of biomass and plastic waste for fuels and chemicals production.
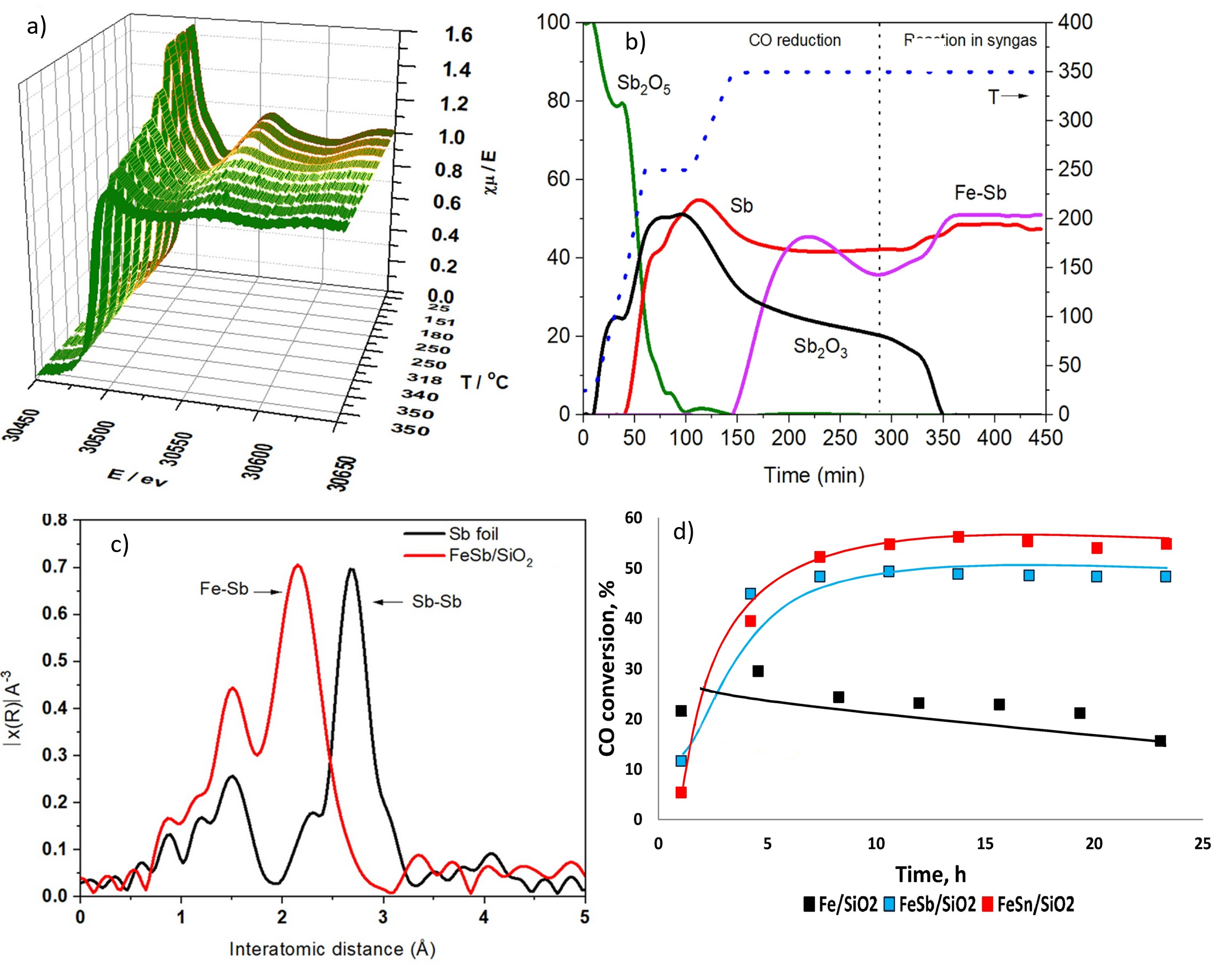
The studies performed in this work enable a clear understanding of the catalytic phenomena and open perspectives for the catalyst improvements. In-situ X-ray absorption spectroscopy performed at the CLÆSS beamline of the ALBA Synchrotron played a crucial role to study the catalysts and promoters under reaction conditions.
The Fischer-Tropsch process is a catalytic chemical reaction first developed in Germany in 1925, in which carbon monoxide and hydrogen in a fuel gas known as syngas are converted into hydrocarbons of various molecular weights. Nowadays, the Fischer-Tropsch synthesis is an attractive way to convert the syngas generated from non-petroleum and renewable feedstocks such as biomass, plastic and organic waste into fuels and chemicals.
One kind of hydrocarbons that can be synthesized by the Fischer-Tropsch reaction are olefins, which are used as building block materials for many products, including plastics, detergents and adhesives.
Researchers from the University of Lille (France) have developed a new procedure for light olefins synthesis using Fischer-Tropsch reaction. They added tin and antimony to the iron catalysts, to increase their activity. This process, called promotion, resulted in a major increase in the reaction rate.
The goal of this work is to elucidate the genesis and evolution of active phases – the ones that present the catalytic properties - in the catalysts during their activation and catalytic reaction using a combination of in-situ and advanced characterization techniques. In-situ experiments, specifically, played a crucial role to study both iron catalysts and promoters, tin and antimony, under reaction conditions.
The studies performed in this work enable a clear understanding of the catalytic phenomena and open perspectives for the catalyst improvements.

Figure: STEM-HADDF and STEM-EDX mapping of the silica supported-FeSb catalyst after activation in CO showing the formation of Fe-Sb core-shell structures.
A closer look into the promoters and catalysts interaction
In-situ X-ray absorption spectroscopy performed at the CLÆSS beamline of the ALBA Synchrotron demonstrated that antimony is completely reduced to the metallic state and forms iron-antimony bimetallic nanoparticles under the reaction conditions, while a significant fraction of tin oxide is still present.
The promotion with antimony results in higher enhancement in iron carbidization (the process of carbides adhering to the iron) compared to promotion with tin. The enhancement of the reaction rate was attributed to the electronic effects arising from the promoters localized in close proximity to the iron carbide nanoparticles. Electronic effects enhance the intrinsic activity of the active sites, which can be thought of as the ensemble of atoms that directly catalyzes a reaction. The specific activity of the active sites, called turnover frequency, increases 7–10 times due to the interaction of iron carbide species with antimony and tin.
This study is a collaboration between researchers from the University of Lille (France), the Delft University of Technology (Netherlands), the Ghent University (Belgium), the ALBA Synchrotron, the French National Centre for Scientific Research (CNRS) and the Normandy University & INSA of Rouen (France).
With the collaboration of Fundación Española para la Ciencia y la Tecnología. The ALBA Synchrotron is part of the UCCs of the FECYT and has received support through the FCT-20-15798 project.