ALBA Synchrotron
Researchers have found that addition of paclitaxel (a type of antitumoral drug) to microtubules alters their structure. This compound modulates the material properties of microtubules by converting destabilized growing microtubule ends into regions resistant to depolymerisation, eventually leading to cell death. Results were obtained at the NCD beamline of the ALBA Synchrotron.
Paclitaxel, one of the most commonly used antitumoural drugs, modulates microtubules, the biopolymers responsible for many essential cellular functions including cell division, movement and intracellular transport. This kind of drugs target tubulin subunits, the main microtubule proteins, and interfere with their dynamics, which can have the effect of stopping a cell cycle and can lead to programmed cell death or apoptosis.
A group of Centro de Investigaciones Biológicas (CIB-CSIC), Madrid, in collaboration with the University of Utrech and the Institute of Materia Medica, Beijing, have found in the ALBA Synchrotron that addition of paclitaxel to microtubules alters their structure, which is the basis for the change in the properties of the microtubule regions to which the drug binds.
Microtubules can dynamically switch between growing (polymerization) and shrinking (depolymerization), a phenomenon known as dynamic instability. This refers to the coexistence of assembly and disassembly of tubulin dimers at the ends of the microtubule.
The work aimed to understand why paclitaxel is able to modulate microtubule function at minimal concentrations, much lower than that of tubulin. Results showed that taxanes modulate the material properties of microtubules by converting destabilized growing microtubule ends into regions resistant to depolymerization, thus stabilizing them.
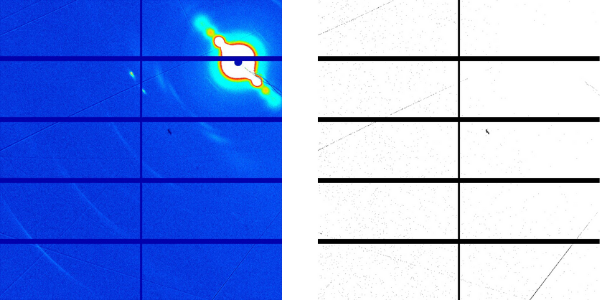
High-resolution fiber diffraction images of microtubules before and after paclitaxel addition were obtained at the NCD-SWEET beamline at ALBA, employing a device developed in collaboration with Prof. Shinji Kamimura from the University of Chuo, Japan. The high photon-flux available at NCD-SWEET combined with the capacity of the developed device to obtain highly aligned microtubule fibers, allowed CIB researchers to determine structural parameters of microtubules with sub Angstrom (Å) resolution.
Based on this information and on the recent work also developed in ALBA researchers can understand the reasons for the paclitaxel induced peripheral neurotoxicity, its main secondary effect and design strategies to minimize it.
Understanding how these compounds modulate microtubules is crucial to designing more effective drugs that overcome cell resistance and lower the toxicity of compounds in clinical use.

Figure: Microtubules are hollow tubular structures found within cells. They are formed from heterodimers alpha- and beta-tubulin. Anti-cancer drugs target these structures by either preventing their assembly or disassembly. This leads to defective mitosis. Credit: Simon Caulton / CC-BY-SA-3.0
With the collaboration of Fundación Española para la Ciencia y la Tecnología. The ALBA Synchrotron is part of the of the of UCC network from the FECYT and has received support through the FCT-20-15798 project.